It’s a wonderful world — and universe — out there.
Come explore with us!

Science News Explores
Explainer: how heat moves.
Here are the three processes by which energy can be transferred from one place to another

Heat is being transferred from the hot end of this rod to the cold end via conduction, but the hot end of the rod is also radiating heat via that orange glow.
Dvoinik/iStockphoto
Share this:
- Google Classroom
By Sid Perkins
September 30, 2016 at 6:15 am
Throughout the universe, it’s natural for energy to flow from one place to another. And unless people interfere, thermal energy — or heat — naturally flows in one direction only: from hot toward cold.
Heat moves naturally by any of three means. The processes are known as conduction, convection and radiation. Sometimes more than one may occur at the same time.
First, a little background. All matter is made from atoms — either single ones or those bonded in groups known as molecules. These atoms and molecules are always in motion. If they have the same mass, hot atoms and molecules move, on average, faster than cold ones. Even if atoms are locked in a solid, they still vibrate back and forth around some average position.
In a liquid, atoms and molecules are free to flow from place to place. Within a gas, they are even more free to move and will completely spread out within the volume in which they are trapped.
Some of the most easily understood examples of heat flow occur in your kitchen.
Put a pan on a stovetop and turn on the heat. The metal sitting over the burner will be the first part of the pan to get hot. Atoms in the pan’s bottom will start to vibrate faster as they warm. They also vibrate farther back and forth from their average position. As they bump into their neighbors, they share with that neighbor some of their energy. (Think of this as a very tiny version of a cue ball slamming into other balls during a game of billiards. The target balls, previously sitting still, gain some of the cue ball’s energy and move.)
As a result of collisions with their warmer neighbors, atoms start moving faster. In other words, they are now warming. These atoms, in turn, transfer some of their increased energy to neighbors even farther from the original source of heat. This conduction of heat through a solid metal is how the handle of a pan gets hot even though it may be nowhere near the source of heat.
Convection occurs when a material is free to move, such as a liquid or a gas. Again, consider a pan on the stove. Put water in the pan, then turn on the heat. As the pan gets hot, some of that heat transfers to the molecules of water sitting on the bottom of the pan via conduction. That speeds up the motion of those water molecules — they are warming.

As the water warms, it now begins to expand. That makes it less dense. It rises above denser water, carrying away heat from the bottom of the pan. Cooler water flows down to take its place next to the hot bottom of the pan. As this water warms, it expands and rises, ferrying its newly-gained energy with it. In short order, a circular flow of rising warm water and falling cooler water sets up. This circular pattern of heat transfer is known as convection .
It’s also what largely warms food in an oven. Air that’s warmed by a heating element or gas flames at the top or bottom of the oven carries that heat to the central zone where the food sits.
Air that’s warmed at Earth’s surface expands and rises just like the water in the pan on the stove. Large birds such as frigate birds (and human flyers riding engineless gliders) often ride these thermals — rising blobs of air — to gain altitude without using any energy of their own. In the ocean, convection caused by heating and cooling helps to drive ocean currents. These currents move water around the globe.
The third type of energy transfer is in some ways the most unusual. It can move through materials — or in the absence of them. This is radiation.
Consider visible light, a form of radiation. It passes through some types of glass and plastic. X-rays, another form of radiation, readily pass through flesh but are largely blocked by bone. Radio waves pass through the walls of your home to reach the antenna on your stereo. Infrared radiation, or heat, passes through the air from fireplaces and light bulbs. But unlike conduction and convection, radiation doesn’t require a material to transfer its energy. Light, X-rays, infrared waves and radio waves all travel to Earth from the far reaches of the universe. Those forms of radiation will pass through plenty of empty space along the way.
X-rays, visible light, infrared radiation, radio waves are all different forms of electromagnetic radiation . Each type of radiation falls into a particular band of wavelengths. Those types differ in the amount of energy they have. In general, the longer the wavelength, the lower the frequency of a particular type of radiation and the less energy it will carry.
To complicate things, it’s important to note that more than one form of heat transfer may occur at the same time. A stove’s burner not only heats a pan but also the nearby air and makes it less dense. That carries warmth upward via convection. But the burner also radiates heat as infrared waves, making things nearby warm up. And if you’re using a cast-iron skillet to cook a tasty meal, be sure to grab the handle with a potholder: It’s gonna be hot, thanks to conduction!
More Stories from Science News Explores on Physics

New lab trick makes diamonds without extreme pressure

Experiment: How to make the boldest, brightest tie-dye!

Flowers may electrically detect bees buzzing nearby
Here’s why scientists want a good quantum computer

Aerodynamics involved in shooting hoops can make vehicles greener
A bit of electricity can glue hard metals to soft materials
The movie Frozen inspired the icy, 3-D printing of blood vessels

Experiment: Make your own cents-able battery

Chapter 6, Heat: The flow of energy and the direction of time

Water, air and temperature
If you take a quantity of water, place it in a cooking pot and turn on the heat, as in this photo, it will warm up and eventually boil. If you keep the heat on long enough, you will boil away all the water. But what happens to that water? Does it stop being H 2 O, two hydrogen atoms bonded with an oxygen atom? Or is it a gas, a vapor, floating around in the atmosphere? And why does a set amount of tap water, let’s say a quart of tap water, take less time to boil if the stove burner is set to “HIGH” than the same amount of water from the same tap but with the stove set to “MEDIUM” heat?

Why do thunderstorms form so often on hot, humid summer days in Florida, as in this photo, above, but not on equally hot days in April when the atmosphere is less humid? A good example from 2016 is the two days April 1 and May 17: high temperatures of 91º F, thunderstorms and over an inch of rain in Orlando on May 17, but no precipitation on April 1. Why was that?

When a hurricane approaches Florida, we listen to weather reports about the central pressure, maybe 900 millibars, we track the storm by satellite, as in this image, right, and we predict its path. But why do we fear elevated surface temperature in the Atlantic and the Gulf of Mexico?
We can apply our ideas of momentum and energy, force and acceleration to all of these questions about the thermal state of water and air. Our applications lead us to extract more advanced concepts like temperature, pressure and heat transport in this chapter.
Solid water, liquid water and gaseous water
The behavior of water at its freezing point and at its boiling point define the set points of the Celsius and Fahrenheit temperature scales.
The third common temperature scale, the Kelvin scale or absolute scale, will become especially important for us later in this chapter when we make calculations. With Fahrenheit and Celsius, however, these phase transitions of water are the anchors to the temperature scale.
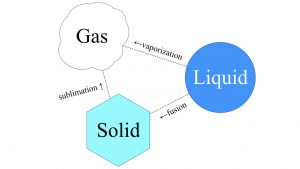
Phase transition is the scientific term for the process in which a liquid form of matter transforms to either a solid or a gas. The words we use for the phase transition from liquid to solid is freeze or solidify or fusion. For instance, a volcano’s molten lava flows like a liquid, but you wouldn’t really say it freezes, — you would say it solidifies. From liquid to gaseous state, that’s either boiling or vaporizing something. Solid matter can also go to the other two phases of matter, liquid and gaseous. Solid to gaseous phase transition is known as sublimation. That’s something that CO 2 , carbon dioxide, does under certain conditions. The polar ice caps of Mars are mostly CO 2 ice. If you go to Publix and buy a big block of dry ice, carbon dioxide ice, it will not leave a pool of liquid when it heats up. That is sublimation. It will go directly to a gas. That’s why we call it dry ice.
Scientists have measured the freezing, boiling points and sublimation of many substances like gold, iron, CO 2 , H 2 O. Here is a short table of freezing and melting points for a few common substances, and, for reference, their densities in solid phase and a few densities in liquid phase. Gases are compressible, so their density varies with pressure and temperature, and they are not amenable to a short table. Note: ammonia is a gas at room temperature, although we purchase it at the supermarket as a bottle of aqueous solution. Also, the densities of liquids are measurements at the boiling point of each liquid.
An example of gaseous to liquid phase transition is water vapor in the atmosphere turning into droplets of rain. At the right altitude, the temperature and atmospheric pressure are just right for water vapor to condense into micro-droplets of liquid water. This forms clouds, a mist of micro-droplets that are so small, they are lofted into the atmosphere by thermal updrafts. That’s why thunder clouds build higher and higher, bigger and bigger. These micro-droplets collide and coalesce into bigger droplets. Eventually they become so big, possibly at a different altitude, and, again, where the temperature and atmospheric pressure are just right, thermal updrafts can no longer hold them aloft. They fall as rain. Whenever you see a cloud, you’re usually looking at liquid droplets of water that are still held aloft. An exception to that is a cirrus cloud, the really high, really wispy ones that look like strands of hair or horses’ tails. Those are actually ice crystals, and on a cold clear night, when the moon is full, ice crystals aloft form a set of rings around the moon, a lovely sight!

One way to think about the three phases is the relative energy rankings. A gaseous material is going to have a higher average energy, atom for atom, molecule for molecule, than the liquid state. The solid state of the same material occupies the lowest of the average energy states. In the solid, the molecules have so little kinetic energy that they’re still stuck in a crystal lattice of some kind, either a diamond crystal or hexagonal crystals like ice, or any other kind of crystal that you may find.
Another factor that affects phase transitions is the ambient pressure. If you put some ribs in a pressure cooker, they cook to a state of tenderness much more rapidly than in a conventional oven or on a barbecue grill, and cooking is a way of making chemical reactions happen. Phase transitions in carbon dioxide display some interesting effects of ambient pressure. Dry ice actually can go to liquid, but only in high pressure environments. We call it dry ice because in normal atmospheric pressure at the surface of earth, it goes straight from solid to a gas. It sublimates. It doesn’t go through the liquid phase the way water does. But you can get solid to liquid to gas from CO2. In other words, you can get a CO2 puddle if the ambient pressure is high enough.
Pressure in a fluid and momentum states of atoms and molecules
The ancient Greeks thought of atoms but not molecules as we think of molecules today. In the 1700s scientists got more serious about the idea of atoms and molecules, and they developed the molecular theory and, as a subset, the kinetic theory of gases. Some of the assumptions of the kinetic theory of gases are
- that an everyday sized sample, i.e., a macroscopic sample like a balloon or a ballroom, has a very large number of particles in it, but that the particles are so small their separations are much larger than the particle size;
- that the particles move in random directions with a huge variety of speeds;
- that the particles do not experience long range interactions but interact by collision with each other and with the boundaries of the container like billiard balls;
- and that the particles obey Newton’s three laws of motion.
This set of assumptions is not meant to be exact and completely accurate, but it is close enough to a real gas that a scientist can use it as a shorthand version, an idealized gas model, from which it is easy to extract macroscopic averages, like a pressure.
The basic idea of pressure, one of a fluid’s measurable physical properties, is that it sums up the effect of all the atoms or molecules exchanging momentum with the sides of the fluid’s container. This diagram shows a perspective view and an overhead view of one such collision.
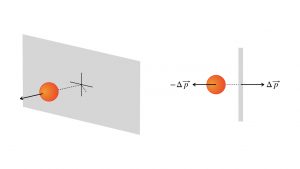
That is, pressure is measured in Newtons per square meter, one number that encodes the effect of many particles exchanging momentum with the wall at a given rate, per second, and at a given density, per square meter. In the metric system, 1.00 N/m2 is named the Pascal, abbreviated Pa, after the French scientist Blaise Pascal who developed the theory of atmospheric pressure and fluid pressures in general. In the customary English system, the pressure measurement is PSI, pounds of force per square inch. In the USA, bicycle and automobile tires have PSI ratings for inflation, about 50 PSI or so for a mountain bike’s tire, about 30 PSI for automobiles. This rating is the amount of extra pressure the air pump delivers above the atmospheric pressure. On television, the weather reports refer to the atmospheric pressure in millibars: 1.000 bar = 100,000 Pa; 1.000 millibar = 100 Pa.
The atmosphere of Earth has mass and weight, approximately 14.7 PSI, 101325 Pa, 1013.25 millibars at sea level on a fair day. Foul weather is usually a function of low atmospheric pressure. For example, a hurricane’s central pressure, in the eye of the hurricane, might be as low 880 millibars or even lower. A fair day in Denver, however, might be about 850 millibars, a lower atmospheric pressure because of the mile high altitude there. That is, the most dense layer of Earth’s atmosphere is below Denver and does not weigh down on Denver’s air.
The atmosphere, abbreviated atm, is another common pressure unit. Exactly 1.00 atm = 101325 Pa. A SCUBA diver might think in terms of atmospheres: every 10 meters of depth underwater adds another atmosphere of pressure from the weight of the water.
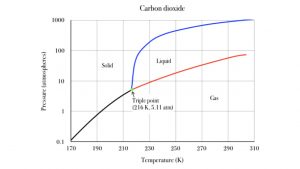
At normal atmospheric pressure, carbon dioxide ice sublimates to carbon dioxide gas. But at high pressure, it can melt to liquid carbon dioxide. The following graph shows the pressure and temperature conditions for sold, liquid and gas phases of carbon dioxide. The black line is the sublimation line. Pressure and temperature conditions divide along the black line, solid to the left of it, gas to the right. If you heat up your sample of solid carbon dioxide, its temperature moves to the right, across the black line, and the carbon dioxide sublimates to the gaseous state. The blue line is the melting line, separating solid from liquid states. Note that this transition only occurs at relatively high pressure conditions, above 5.11 atmospheres! That is over five times more pressure than our atmosphere provides. The red line divides liquid from gas: this is the evaporation line. Heating liquid CO 2 from left of the red line moves it across to the gas phase. This is also a special pressure effect above 5.11 atm. The 1 atm line is quite a bit below the CO 2 triple point.
The green triple point represents the unique temperature and pressure condition at which carbon dioxide is indifferent to solid, liquid and gas. They coexist at the temperature and pressure. Similarly, for water, solid and liquid H 2 O are thermally indifferent to each other when their temperature is 0°C. This is ice water! In a well insulated thermos container, which prevents heat energy from entering or leaving, the liquid grams of water will stay liquid for a long time, without freezing, and the solid grams of H 2 O ice will stay solid for a long time, without melting.
Temperature in a substance and kinetic energy states of atoms and molecules
has a square root of the molecular mass m in its denominator. When you use a few trigonometric, calculus and statistics techniques, applying Newton’s laws carefully, you also find that the temperature is proportional to the average kinetic energy. The temperature-energy relation is quite simple:
E pluribus unum and the ideal gas
Now we have two macroscopically observable properties, temperature and pressure, that are direct results of the submicroscopic energy and momentum states of all the gas particles we cannot see. As in the motto of the United States, e pluribus unum, “out of many, one,” so with kinetic theory, from many submicroscopic particles, we extract one energy parameter, the temperature, and one momentum parameter, the pressure. This ideal gas model is useful, because a real gas measured in the lab is very close in its properties to the ideal gas model.
Liquid water and the definition of the calorie
One of the metric system definitions of energy is the calorie. It is different from the Joule, but one can use calories wherever one uses Joules. A thermochemical calorie is defined as the amount of energy required to heat up 1.00 gram of liquid water from 14.5°C to 15.5°C, at 1.00 atmosphere of ambient pressure — i.e., at sea level on a fair day. One thermochemical calorie, 1.00 cal, is equivalent to 4.184 Joules. This is different from the conventional food calorie, which customarily uses a capital C and is equivalent to one thousand thermochemical calories: 1000 cal = 1.000 Cal. These big calories appear in nutrition facts on food packaging, such as the Twinkies nutrition fact, 150 calories for one serving. Twinkies notwithstanding, we will do almost all of our thermal calculations in thermochemical calories.
How do you heat up carbon dioxide and other substances?

The formula for computing heating up or cooling down energy Q is just the product of the three physical quantities, viz.
For the remainder of the chapter, we will keep to the Kelvin scale. It is a metric system scale, but oriented to the kinetic theory, not the behavior of H 2 O. The Celsius scale goes to zero when water freezes, but the Kelvin scale goes to zero at the theoretical point where molecular kinetic energy is zero.The size of a Kelvin degree, 1.00 K, is the same size as a Celsius degree, 1 C°. The zero point of the Kelvin scale is deep in the negatives of the Celsius scale, -273°C. The freezing point of H 2 O is 273.15 Celsius-sized degrees above the zero point at T = 0.00 K.
Note: it is customary to state the units of Kelvin temperature with a capital K but without the ° degree symbol. There are still 100 degrees of separation between H 2 O freezing and boiling points, but the scales are labeled with different numerals, 0 for Celsius, 273.15 for Kelvin at the freezing point.
Working out a few realistic examples of heating and cooling

As a second example, let’s work with solid H 2 O, ice. Let’s take a 350 g slab of ice out of a commercial freezer that is set to 0°F, or 255 K. We want to heat it up to the melting point, 273 K. But this is solid water, its molecules more organized than the same amount of liquid H 2 O, so the ice is actually easier to heat up.

So even though each gram is relatively easy to heat up by one Kelvin, there are so many grams to heat up that our energy budget for this process, Q, must be quite large:
How to melt ice, aluminum and anything else
Now let’s look at the energy requirements for actually melting solid H 2 O into liquid H 2 O, or solid aluminum into liquid aluminum. Once you have heated your substance to the melting point, the intermolecular bonding will begin to degrade. The molecules, on average, have attained an elevated energy state from which their intermolecular bonds are vulnerable, for instance the crystalline bonds of H 2 O ice or the metallic bonds of aluminum. After crystalline or metallic bonds have broken down, neighboring molecules might still experience weak interactions, as a liquid, strong enough to keep a droplet round and not randomly shaped, but not strong enough to reforge good strong crystalline or metallic bonds. That is, their average kinetic energy is too high to get a pervasive, strong lockdown crystalline bond or metallic bond. A similar logic applies to the vaporization phase transition between a liquid, loosely bonded molecules, to a gas, no bonds of any kind, complete chaos.
But how much energy does it take to degrade the bonds? The degrading goes pair by pair of molecules, so it depends entirely on how many molecules are in your sample. A scientist might measure this in grams or kilograms. And the energy requirements differ for each phase transition, whether you are breaking down a solid or breaking down a liquid. Scientists measure various substance carefully by isolating a liquid and letting it evaporate, or by taking solids and melting them. The amount of energy measured per gram of substance is called the latent heat. In addition, for each substance, there is a latent heat of fusion (melting or freezing) and a latent heat of vaporization (boiling or condensing). Here is a small table of some latent heats.

Notice how much easier lead is to melt, compared to H 2 O ice, gram for gram. Gold is also relatively easy to melt.
One of the interesting properties of matter is connected to latent heat. That is, when you heat up a sample, its temperature increases until it gets to the melting point, but then it is stuck at the melting point as you continue to pour on the calories. The process of melting takes over, so each calorie breaks apart the solid into its liquid state. Once all of the solid is melted, then the liquid’s temperature begins to rise. Ice water is an example of this. Theoretically, you can use a beaker of ice water to keep a test tube of any substance at 273 K, as long as the ice remains. If your ice water container is fairly well insulated, this could last for quite a while.
Heat transport
There are three ways heat energy flows from point A to point B: conduction, convection and radiation . There is only one condition for heat to flow from point A to point B: if the temperatures are different at each point. To determine if two quantities of matter are in thermal equilibrium, one must look at the two temperatures.
- If the two temperatures are equal, they are in thermal equilibrium.
- If the two temperatures are unequal, one will be larger, the other smaller, and heat energy will flow from the larger temperature substance to the lower temperature substance.
Two good example of this condition, unequal temperature, are well-known to doctors and nurses in the emergency room:
- A swimmer in the ocean, without a wetsuit, can develop hypothermia very rapidly, and the emergency room will place warm compresses on the swimmers neck, chest and lower abdomen, to warm up the body core. Advanced treatment includes blood rewarming, with the patient’s blood circulating through a dialysis machine or similar device. These treatments cause heat energy to flow from external hot objects or rewarmed blood into the colder body core.
- Against this example, you may also consider a case of heat stroke . One of the emergency treatments for heat stroke is immersion in a bath of ice water! In this case, heat flows from the patients body into the bath of ice water.
In both of the examples, hypothermia and heat stroke, the direction of heat energy is from higher temperature to lower temperature, and the transport mode is conduction. That is, the hot compresses, the rewarmed blood, the heat stroke patient, are actually in contact with the colder substance, and heat energy diffuses across the boundary between the two. In the emergency room, the doctors and nurses monitor the patient’s temperature carefully. They do not want any random equilibrium temperature: they want the patient to force the patient’s temperature to 98.6°F, 37°C, 310 K. Then, as they trust and hope, the patient’s normal biological function maintains stable body temperature.
In a laboratory, you might allow two gases to interact and reach their own equilibrium temperature, without forcing. For example, you could take a warm gas at initial temperature 500 K, in an insulated container, and another gas at initial temperature 400 K, in an insulated container.
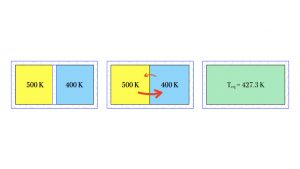
If you remove the insulating wall separating the two samples, the gases begin to mix and equilibrate. The average kinetic energy of the molecules of warmer gas move into the cooler region more rapidly than the poky molecules in the cooler gas, which have lower average kinetic energy. It is true that the poky molecules from the cooler gas do spread to the warmer region, but they do not do so very rapidly, due to pokiness — i.e., due to lower average kinetic energy. The diagram above shows this in the two red arrows: larger and thicker for the blazing average kinetic energy of the molecules of the warmer gas, and a smaller, thinner arrow for the poky average kinetic energy of the cooler gas. So, thinking in terms of kinetic energies, more net kinetic energy crosses from the warm side to the cool side. The hot side might still read a relatively higher temperature, but its average kinetic energy is decreasing due to a higher number of poky molecules, so its temperature might dip to 499 K in the first second of mixing. Similarly, the cool side is hotting up, due to the arrival of molecules with high average kinetic energy, and its temperature might blaze upward to 400.3 K in that same first second of interaction.
Eventually, the molecules of each gas mix so thoroughly that the entire sample of the mixture has arrived at one temperature, the equilibrium temperature. In the diagram above, the equilibrium temperature is 427.3 K. Is it plausible that the equilibrium temperature is not exactly halfway between the two initial temperatures? Yes, it might be off center, because the two gases
- might be different gases, which might mean different specific heats, and
- might be different amounts, more grams of the cooler gas, for instance, and
- in their separate containers, their initial pressures, which we did not mention, might also have been different.
Even if everything is different — different specific heats, different amounts, different pressures — they will attain an equilibrium temperature somewhere below the hotter gas’ initial temperature and above the cooler gas’ initial temperature.

Convection is a heat transport method that tends to proceed in a fluid that is not a very good conductor of heat. Instead of conducting, a bolus of warm material rises through cooler material, without losing much energy by conduction. This transports heat energy. An example is a fire breather. He spews liquid paraffin out and up, and ignites it (by conduction!) with a small side torch. Instead of arcing across a parabolic path, however, the mass of ignited paraffin heats up the air and the entire fireball lifts upward. The mass of high temperature fuel-air mixture moves from point A upward to point B. This only works with cooler surrounding air, which is more dense. The ignited fuel-air mixture is at a lower density, and buoyant forces give it some upwards F = ma force.
A similar example of hot air rising, cool air descending is a hot air balloon. They work best when the surrounding air is cool, in the early morning. The buoyancy force on the hot air in the balloons lofts the entire balloon.

But once aloft, they can stay aloft by periodically burning their propane upward into the balloon. If they need to descent back to terra firma, they just vent the balloon, less hot air, left buoyancy force, net force downward.
All of this works because air is a poor conductor of heat, or, as we might say, it is a good insulator. You can understand this by looking at a fleece vest or a fur hat or soft nice winter gloves or a thick, comfy scarf. The fibers have many small air spaces between them, trapping air, a good insulator. Being trapped, the air cannot convect and move warm air toward cooler air, keeping you warm and comfy. Some fibers actually contain tiny air pockets, further increasing the insulating power. Alpacas might be funny looking, but do not make fun of them, because their hair is hollow, keeping them warm and happy at the high altitudes of the Peruvian Andes.

Humans have also figured out how to manufacture hollow polyester fibers, too. Many trademarked names reflect this, such as Hollofil®, a synthetic polyester popular with bedding and pillows. Backpackers use another hollow core fiber, Polarguard® Delta, for very lightweight sleeping bags and parkas. These fibers keep in warmth and are not rendered useless by getting soaked with water. Goose down is the best insulator for very cold conditions. However, goose down has to stay dry, because it tends to hold onto water, and water is a relatively good conductor of heat.
The concept of radiation as a heat transport method, is based on what we know about light. Each photon of light carries energy and momentum.
- A substance that absorbs the photon’s energy and momentum will tend to heat up.
- A substance that is transparent to light, does not absorb much energy and does not heat up very much at all.
Our planet is warm because of radiative heat transport from the photosphere of the Sun, the outermost layer of the Sun from which most visible light is emitted.

But interplanetary space is quite happy to grant photons a free passage, so most of the light aimed toward Earth actually does arrive here. It warms the oceans and the atmosphere and all of the water vapor in the atmosphere. Most of our weather depends on solar radiation. Anyone living in Florida knows that the surface temperature has a dramatic effect on the intensity of hurricanes.
Example 1. Forty grams of H2O ice

A 40 g sample of H 2 O ice at initial temperature 253 K must be converted to steam at final temperature 393 K.
What is the energy budget, total energy required, for this process?
This heat-melt-heat-boil-heat process takes the 40 grams of H 2 O through both phase transitions. It also heats the H 2 O in each phase. In list form, it is
- heat the ice up to 273 K, the melting point;
- melt all 40 grams of ice at 273 K;
- heat the liquid H 2 O from 273 K up to the boiling point, 373 K;
- boil the 40 g of H 2 O to form 40 g of steam at 373;
- heat up the steam to the target temperature of 393 K.
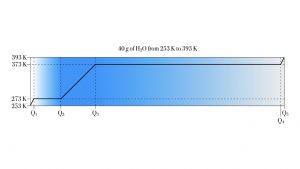
Let’s work out each part of the energy budget, stage by stage.
Example 2. Finding an equilibrium temperature for a mixture of fluids.
In a well insulated container, you mix 20 grams of liquid water, initially at temperature 290.00 K, with a 40 gram squirt of ethanol, initially at a colder temperature, 275.00 K.
Find the equilibrium temperature.
In this process, the liquid ethanol will absorb energy from the water. Since the container is insulated, the ethanol is the only thing that can absorb energy from the water. This is actually another example of the conservation of total energy, this time including the heat energy. Therefore the ethanol’s equilibrium temperature will be above 275 K. Also, every calorie of energy that the water loses also serves to decrease its temperature. Therefore the equilibrium temperature will be below 290 K. The water cools down, energy flows into the ethanol, and the ethanol warms up. Calorie by calorie, the temperatures change, getting closer for each calorie exchanged.
One can see in this that the ethanol just a bit harder to heat up, calorie for calorie, than the water is to cool down. That is, after exchanging 10 calories, the water cools a bit more than the ethanol heats up.
When the two fluids exchange 10 calories, the temperatures change to 289.50 K for water and 275.43 K for the ethanol. This is level 1 in the sequence. After another exchange of 10 calories, sequence level 2, the water is down to 289.00 K and ethanol is up to 275.86 K. They are closer, but still not at equilibrium. Nature continues to exchange calories of energy, inching closer to equilibrium.
After about 160 calories have flowed out of the water and into the ethanol, their temperatures are both fairly close to 282 K. Therefore, the equilibrium temperature is approximately 282 K.
Note: This is an idealized mixing problem. Real ethanol and water dissolve, and the process of these two compounds dissolving actually gives off energy as heat.
The sequence parameter in the table above is a one-way parameter for both substances. That is, nature does not run in the opposite direction, from cool ethanol to even cooler ethanol and warm water to even warmer water. Nature does not proceed in the manner of energy flowing out of a cool object and into a hot object, if one looks at average temperatures. Nature never does this. It only proceeds by way of energy flowing from a hot object into a cooler object, from a hot collection of particles into a cold collection of particles. This makes the sequence parameter like time itself.
As mathematical physicist John Baez once wrote, “Why is the future so much different from the past?” [1] He noted that we make plans for a time in the future; nobody plans for something that happened last Tuesday. Similarly, we remember past events only; one does not simply remember things that happen next Thursday. We are on a one-way trip into the future, at the speed of 60 minutes per hour, just as the thermal behavior of the water and alcohol is on a one-way trip to thermal equilibrium. This comparison of thermal behavior to our sense of time is called the thermodynamic arrow of time. Neither John Baez nor Albert Einstein nor anyone else has ever explained this scientifically. Time is the deepest riddle of them all.
- Baez, John. "Open Questions in Physics." Last modified: September 21, 2007. http://www.math.ucr.edu/home/baez/open.questions.html ↵
Physical Science Lecture Notes Copyright © 2019 by Thomas J. Brueckner. All Rights Reserved.
Share This Book

Why Does Heat Flow From Hot to Cold?
By: Author Chris Hewitt Reviewed in accordance with our Editorial Policy.
Posted on Last updated: March 23, 2023
Categories FAQ
Ice cream begins to melt as soon as it’s in the cone; chocolate chip cookies start to lose their gooey goodness as soon as they’re out of the oven. But why? Why does everything warm eventually become colder?
Heat flows from hot to cold because warmer molecules move faster. When warmer molecules bump into cooler ones, two things change: cooler molecules speed up (get warmer), and warmer molecules slow down (get cooler.) The flow of kinetic energy from warmer objects to cooler objects is “conduction.”
Keep reading to find out more about this process. Learn what atoms and molecules are, why they’re always moving around, and how heat affects motion. Finally, know why conduction only happens in one direction—why cold objects don’t just keep getting colder.

Table of Contents
Everything Is Made of Particles
The kinetic molecular theory tells us that everything around us is created from tiny particles called atoms . Atoms can join together to make molecules . In fact, most of the substances around us are made of molecules. Our oceans and atmosphere are mostly made up of molecules, and so are we—our DNA, blood, bones, and tissues are all made of molecules.
For more about atoms, see this Khan Academy video:
Here’s a great visualization of how molecules form:
Particles Are Always Moving
We also know from the kinetic molecular theory that atoms and molecules are always moving, unless they’re extremely cold. Any atom or molecule that’s warmer than “ absolute zero ” is in motion. “Absolute zero” is defined as “zero kelvins,” a temperature equal to −459.67° F (−273.15° C). You can see that it’s very unlikely that you will encounter any matter that is at absolute zero, so for all practical purposes, we can say that matter is always moving.
Particles of matter move in three basic ways: they rotate, vibrate, and translate. “Rotate” means spin, “vibrate” means shake, and “translate” means to move from one place to another. The atoms and molecules in the floor underneath you, in the air around you, and even in your own flesh and bones are vibrating, shaking, and translating right now. But when we talk about the movement of heat being due to the movement of particles, it’s really only their vibrating motion that we’re talking about.
Hear Neil deGrasse Tyson explains why absolute zero is more theoretical than practical:
Watch kids perform a basic experiment that demonstrates the motion of matter:
Read more about particle movement in Reactions: An Illustrated Exploration of Elements, Molecules, and Change in the Universe . The photographs in this beautiful book explore how energy, time, and motion create molecular changes in the world around us.
You could also conduct some delicious experiments with molecules by learning about molecular gastronomy, using the Molecule-R – Molecular Gastronomy Starter Kit .
Particles That Move Faster Have More Heat
Applying energy to a substance makes its particles move faster, increasing its heat and temperature. To understand how this works, it helps to know the scientific meanings of heat , energy , and temperature .
- Energy is what needs to be transferred to a substance in order to increase its heat.
- Heat is the total energy contained in the movement of a substance’s particles.
- Temperature is what you get when you divide heat by the number of molecules present in a substance.
This may seem confusing but think of a bowl of soup that’s been heated to 200°F (93°C).
If you scoop some onto a spoon, the soup in the spoon—at least at first—is the same temperature as the soup in the bowl. They’re both 200°F (93°C) because they contain the same average heat per molecule.
But the bowl contains a much larger volume of soup than the spoon does—so the soup in the bowl has more heat than the soup in the spoon because heat is a measure of the total energy contained in the vibration of the soup’s molecules.
If you put the bowl of soup in the microwave, the microwave energy will make its particles move faster, adding more energy to the soup and increasing its heat. A full bowl will take longer to heat to the same temperature than a half-full bowl because the greater number of molecules in the full bowl means more energy is needed to increase the average heat per molecule.
If these terms are still confusing, check out this quick and easy-to-follow video:
Collision Creates Heat Transfer
When two substances are in contact with one another–the air, a bowl, and some hot soup, for example—their atoms and molecules collide as they vibrate. In this collision, energy is transferred, flowing from the substance with the faster molecules and higher temperature to the substance with the slower molecules and lower temperature. That is, heat flows from the hot soup to the air and bowl and from the bowl to the air.
This process of heat transfer between things that touch is called thermal conduction .
To visualize why this happens, think about when another person bumps into you. If you’re both essentially the same size, the person moving more slowly will be knocked farther, faster, of course, than the speedier person.
Thermal conduction doesn’t always occur at the same rate. The thermal conductivity of the two substances can make a big difference—some substances are just better than others at transferring energy. Starting temperatures matter, too. A large temperature difference between the two substances will create faster heat transfer; in addition, some substances become better conductors as they get warmer.
For a visual explanation of the process of thermal conduction, see this video:
You can experiment with thermal conductivity yourself, with the Arbor Scientific Ice Melting Blocks, Thermal Conductivity Experiment Kit , or the Thermal Conductivity Experiment from Eisco Labs .
For some hands-on learning about heat transfer and other physical and chemical properties of matter, try Theodore Gray’s Completely Mad Science: Experiments You Can Do at Home but Probably Shouldn’t .
Heat Transfer Only Works in One Direction
It’s logical to wonder why, in the collision between substances of different temperatures, the colder particles can’t transfer some of their energy to the warmer particles.
We know that this doesn’t happen because when we drop ice cubes into a glass of water, the water always gets cooler and the ice always melts. If conduction could work in the reverse direction, sometimes the water would get warmer, and the ice would become even colder. But knowing that it doesn’t happen isn’t the same as knowing why it doesn’t happen.
This principle—that when two substances are in contact, heat will always flow from the warmer substance to the cooler substance—is called the Second Law of Thermodynamics .
The laws of thermodynamics tell us that something called entropy is at work in the universe. Entropy is a kind of measurement of randomness or disorder, and it can only stay the same or increase—it can never decrease. The Second Law of Thermodynamics tells us that heat will flow from warmer to cooler systems because of entropy—because warmer systems have more entropy.
Whenever two substances touch, the Second Law of Thermodynamics says that heat will flow from the warmer substance to the cooler substance. This happens because the faster-moving particles in the warmer substance collide with the slower-moving particles in the cooler substance.
The collision causes energy to be transferred from the particles of the warmer substance to the particles of the cooler substance. This collision resulting in heat transfer is called conduction.

Chris is a Texas-based freelance writer who loves the outdoors and working in his garage. When he's not enjoying the Texas sun, he can be found tinkering with all sorts of things in his workshop.
View all posts
As an Amazon Associate, we earn from qualifying purchases. We may also earn commissions if you purchase products from other retailers after clicking on a link from our site.
Introduction to Heat Transfer

Hands holding a hot mug (baza178, iStockphoto)
How does this align with my curriculum?
Share on: facebook x/twitter linkedin pinterest.
Learn about the different ways that heat is transferred.
What is heat?
Think about all the ways that you can heat something up. You can boil water on the stove, rub your hands together quickly, or stand in front of a fire. But what is heat ?
Heat is related to thermal energy . Thermal energy comes from the movement of tiny particles inside all matter. All solids, liquids, and gases are made up of small particles such as atoms and molecules. These particles have kinetic energy and are constantly moving. When these particles move more quickly, the amount of thermal energy increases.
Heat is thermal energy that is moving from one place to another. Heat flows from warmer objects to cooler objects. Since heat is a form of energy it is measured in Joules or sometimes in calories .
Misconception Alert Objects don’t contain heat. They can contain thermal energy.
So what’s the difference between heat and temperature ? Temperature tells us how hot or cold something is. Temperature is a measurement of an object’s average kinetic energy. Basically, it is a measure of the average motion of an object’s particles. Temperature is measured in degrees Celsius , degrees Fahrenheit , or using the Kelvin scale. Temperature and heat are connected . Heat is the flow of thermal energy between objects with different temperatures.
Shown is a colour illustration of a pot on a burner over flames. The pot is bright red. Its lid is open and steam is escaping. Across the front are the words, "Temperature is how hot or cold an object is." The pot sits on a black bracket over a burner with five flames. Below, a grey box contains the words, "Heat is transferred to an object. For example how the stove "heats" the pot.
Did you know? A calorie is the amount of energy required to raise the temperature of 1 gram of water by 1 degree Celsius. The energy in the food you eat is measured in calories.
How is heat transferred?
Have you ever held a cup of hot chocolate after being outside in the cold? Holding a hot cup makes your hands feel warmer. What you are experiencing is the transfer of heat from one object to another. Heat energy from the hot chocolate is transferred to your hands.
When two objects have different temperatures, heat is transferred. The cooler object gets warmer until the two objects have the same temperature. Heat energy always flows from the warmer object to the cooler object.
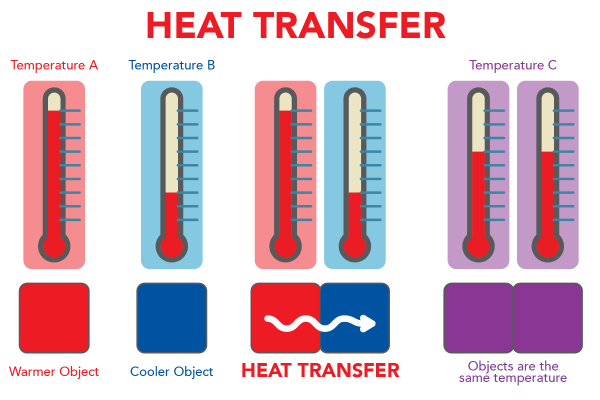
Shown is a colour diagram illustrating the process of heat transfer. The title, Heat Transfer, is in red block letters across the top. Below are three illustrations of the same pair of thermometers above a pair of coloured squares. The first illustration, on the left, shows the two squares quite far apart. The one on the left is red and labelled "Warmer Object." The first thermometer above, labelled "Temperature A," shows red liquid almost to the top. The square on the right is blue and labelled "Cooler Object." The thermometer above, labelled "Temperature B," shows red liquid just above the bottom of the scale. The second illustration shows the squares close together, touching sides. A squiggly white arrow points from the red square to the blue square. This is labelled "Heat Transfer." The thermometers above show the same temperatures as the first illustration. The third illustration shows both squares are now purple. This is labelled "Objects are the same temperature." Above, both thermometers show the same amount of red liquid. These are labelled "Temperature C."
Heat can be transferred in three ways:
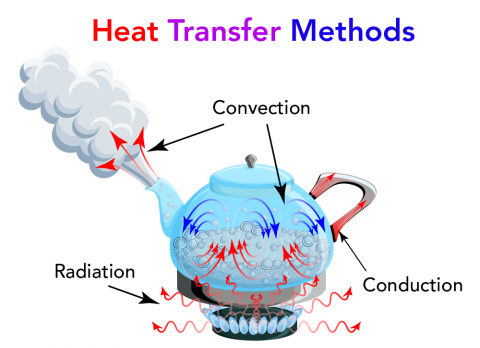
Shown is a colour illustration of three types of heat transfer happening inside a kettle on a stove. Starting at the bottom, blue flames rise from a round black burner. Red squiggly arrows extend above the flames in all directions. Some of these arrows reach through the bottom of the kettle. These are labelled "Radiation." Inside the kettle, the water is bubbling. Small red arrows curve from the bottom of the kettle up to the surface of the water. In the air above the water, small blue arrows curve down into the bubbles. Larger red arrows point out from the spout of the kettle. through steam. All these arrows are labelled "Convection." Another set of red arrows point from inside the kettle, along the handle. This glows red near the kettle sides. This is labelled "Conduction."
Conduction happens when materials or objects are in direct contact with each other. The molecules in the warmer object vibrate faster than the ones in the cooler object. The faster vibrating molecules collide with the slower molecules. This makes the cooler molecules vibrate more quickly, and the object gets warmer. For example, have you ever sat on a cool couch? Did you notice how the seat was much warmer when you stood up? Heat from your skin was transferred to the couch through the vibration of molecules.
Conduction can also happen within a single object. Think of a metal rod that has just been poking around in a fireplace. The end of the rod that’s been touching the hot embers becomes very hot. Energy from the hot end will move through the rod to the colder end. Eventually, the temperature of the entire rod will be the same. This is why it is important to wear a glove when handling a hot metal rod!
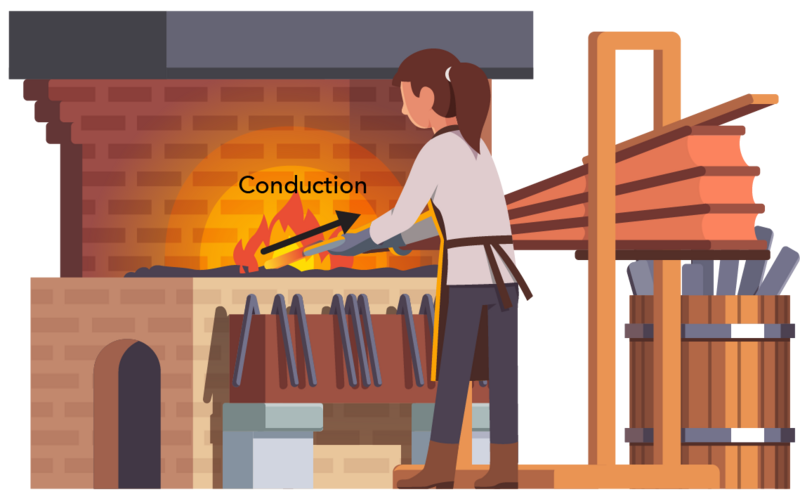
Shown is a colour illustration of a person holding a piece of metal in flames . An arrow pointing from the flames to the metal is labelled "Conduction." The person is shown from the back, wearing a long brown apron and grey gloves. The flames are on a surface about waist height. This is part of a large brick structure that looks similar to an oven. To the right is a large set of bellows and a barrel with more metal bars.
Some materials are better than others at conducting heat. You might have noticed this walking around your house in the winter. Have you ever noticed that your feet get much colder walking on bathroom tile than on carpet? This happens even though both the tile and the carpet are the same temperature as your house. However, tile is a much better conductor than carpet. More heat flows from your foot to the floor when walking on tile than carpet.
Thermal conductivity is a measure of how well a material conducts heat. Materials that are good at conducting heat are known as conductors . Metals, such as silver, copper, and aluminum are conductors. Materials that are not good at conducting heat and are known as insulators . Styrofoam, snow and fiberglass are examples of insulators. Many homes have insulation. Insulation keeps homes from losing too much heat energy to the surrounding air. Many common objects also provide insulation from air such as coolers, insulated flasks and sleeping bags .
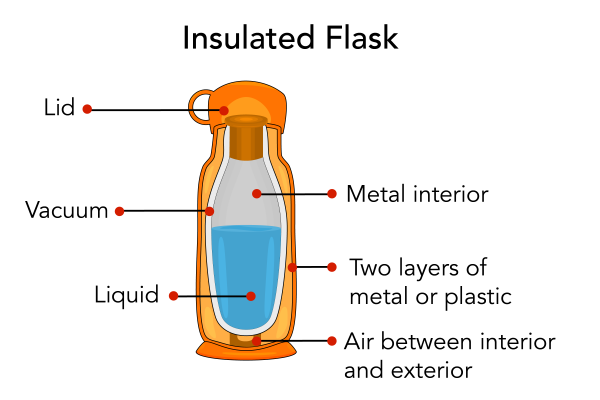
Shown is a colour illustration of the layers inside an insulated flask. The outside of the flask is orange and cylindrical. The inside is smaller and more rounded. The outside wall is labelled "Two layers of metal or plastic." The pale orange space inside that is labelled "Air between interior and exterior." The white space between the double walls of the inside container is labelled "Vacuum." The grey surface inside is labelled "Metal interior." A blue substance inside is labelled "Liquid." At the top is an orange cylinder with a large handle labelled "Lid."
Did you know? Chefs like to use wooden spoons because wood is not a good conductor of heat. This means the spoons won't heat up too quickly and burn their hands.
Conduction usually happens in solids . The particles in liquids or gases are farther apart than in solids. This makes it easier for gas and liquid molecules to move around. Thus, liquids and gases more often transfer heat through convection.
Convection is another way that heat can be transferred. Convection is motion in a gas or liquid that is caused by temperature differences. This motion transfers heat throughout the gas and liquid. The molecules in liquids and gases are farther apart and have more room to move around than in solids. Because of this, heated liquid or gas molecules can physically move. This is different from conduction, where the molecules just vibrate more quickly.
Heating a pot of water on a burner is an example of convection. Heat transfers to water molecules at the bottom of the pot through conduction. These molecules start moving faster. The water at the bottom of the pot becomes less dense. It rises above the denser, cooler water. As the water rises, it carries heat energy upwards with it. Cooler water takes its place at the bottom of the pot where it is heated. This creates a circular cycle of heat transfer. This pattern is known as convection.
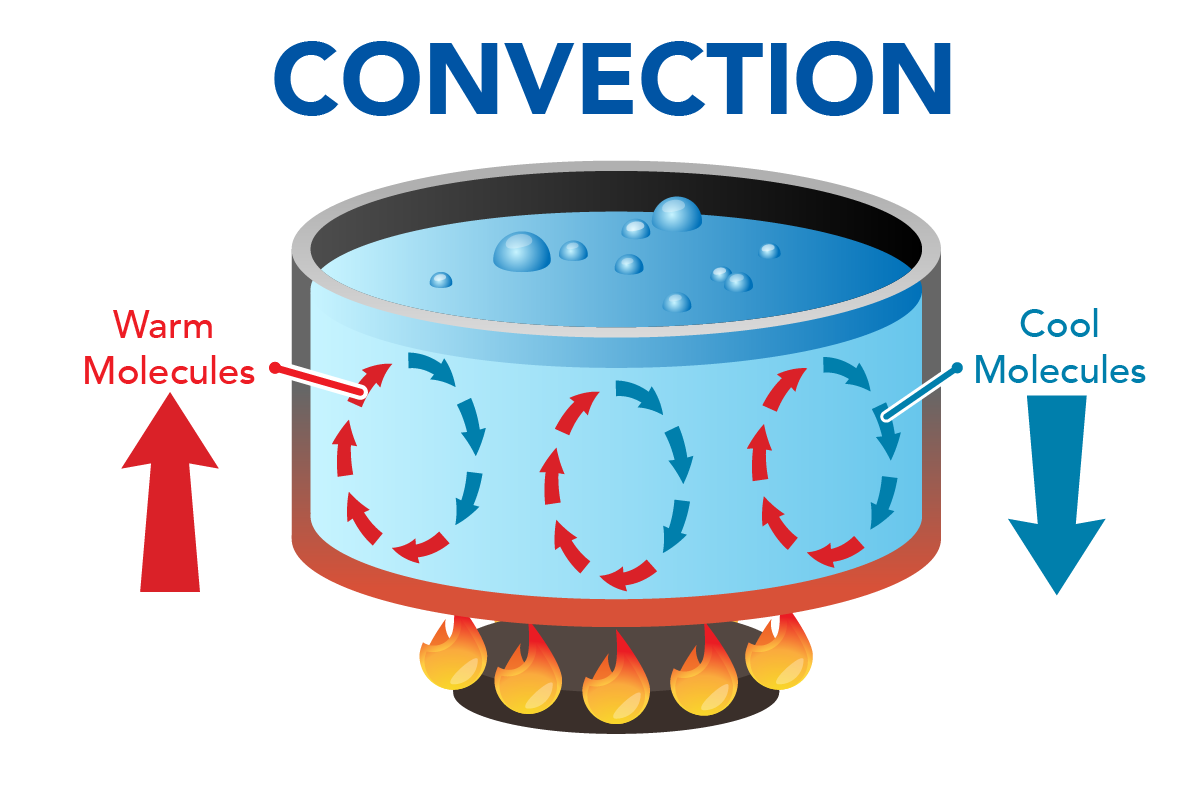
Shown is a colour diagram illustrating the motion of molecules inside a pot of boiling water. The title, "Convection" is in blue block letters across the top of the diagram. In the centre is a cross section of a large pot of pale blue, bubbling liquid. This sits above a round black object with flames around the edge. In the liquid are three vertical ovals of arrows moving clockwise between the surface of the water and the bottom of the pot. Red arrows move from the bottom up. These are labelled "Warm Molecules" with a large red arrow pointing up. Blue arrows move from the surface down. These are labelled "Cool Molecules" with a large blue arrow pointing down.
Convection plays a very important role in wind and ocean currents . For example, air over land is generally warmer than air over the ocean. The warmer air heats up and rises. It is then replaced by cooler air from above the ocean. We experience this movement of air as wind.
Radiation is the third type of heat transfer. Unlike convection and conduction, no matter is needed for radiation. Thermal radiation is the transfer of energy via electromagnetic waves . Electromagnetic waves carry energy across space. Thermal radiation is the way that the Sun heats the Earth. The Sun’s energy travels in waves through space, not through atoms or molecules. Other warm objects, such as a toaster or your body, also radiate heat energy. A microwave also uses radiation to heat your food.

Shown is a colour illustration of radiation waves travelling from the Sun to the surface of the Earth. The Sun and Earth are shown against dark purple and black space with tiny, bright stars. The Sun is in the top left corner. It is a yellow sphere that glows with yellow and orange light. Earth is a bright blue sphere with the continents in bright green. Earth's atmosphere is illustrated with a thick layer of yellow, orange, red and purple around the planet. Long, yellow, wavy arrows stretch from the sun to the surface of the globe. They point to one side of the globe, illuminating the west coast of North America.
Heat Transfer in a House
An example of all three heat transfer processes occurring at the same time is the heating or cooling of a house.
- Conduction can either heat or cool the house. In the summer, heat is transferred from the warm air outside into the house through the walls or roof. In the winter, heat is transferred from the warm air inside the house out through the wall or roof.
- Convection occurs inside each room. Warmer air rises towards the ceiling and cooler air sinks towards the floor. Convection is also why the second floor of a house feels hotter than the basement.
- Thermal radiation from the Sun heats the roof of the house. Radiation can also transfer heat energy through windows.
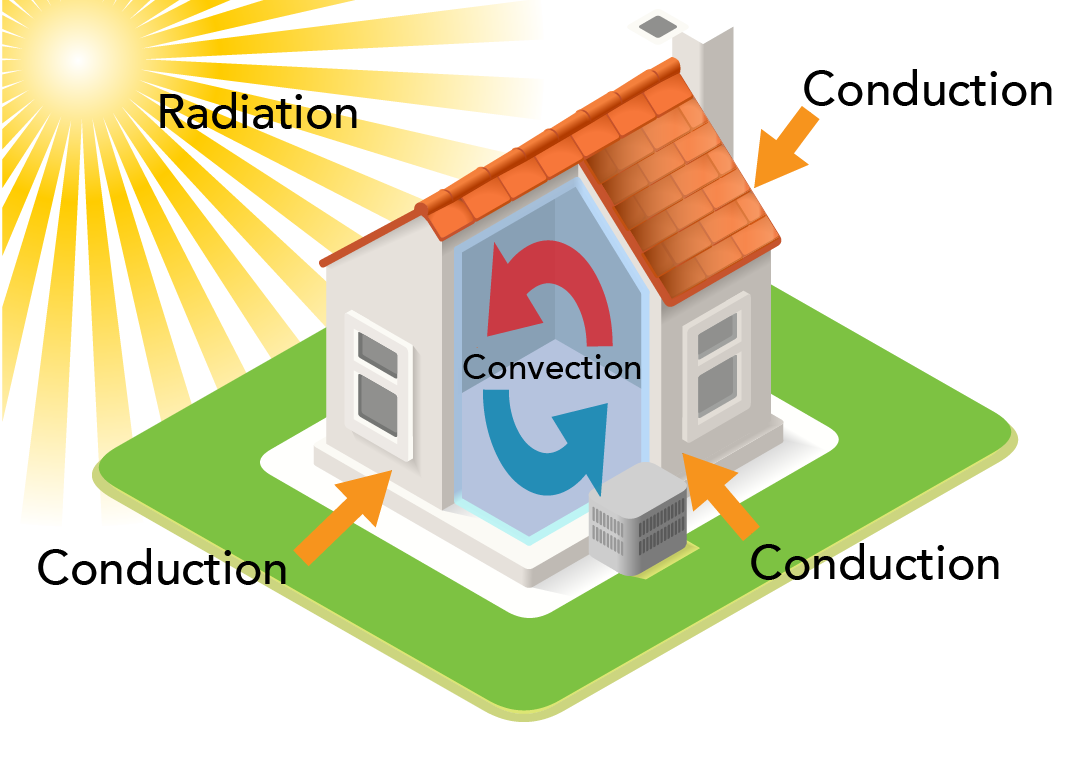
Shown is a colour diagram of a house with arrows indicating how heat is transferred in and around it. The house is small and square in the centre. It has a chimney, a red peaked roof and a strip of green grass around it. One corner is cut away to show the space inside. The sun shines in the top left corner. Its rays are long and yellow, reaching down to the house. This is labelled "Radiation." Three orange arrows point from outside the house to the walls and windows. These are labelled "Conduction." Inside the house, a red arrow curves up across the ceiling and down the wall. A blue arrow curves across the floor and up the wall. These are labelled "Convection.
We experience these different forms of heat transfer everyday. Understanding these concepts can lead to innovative uses of heat energy. For example, a Canadian teenager created a flashlight powered by the heat of your hand . Who knows what other ways we will use our knowledge of heat in the future.
Thermal Imaging Learn more about thermal imaging in this article by Let’s Talk Science.
Heat Transfer: Crash Course Engineering #14 This video (8:35 min.) from PBS explains heat transfer and the different mechanisms behind it.
Heat Capacity, Specific Heat, and Calorimetry This video (4:13 min.) by Professor Dave explains how we can measure temperature changes.
There's No Such Thing As Cold (2015) This video from It's Okay to Be Smart (5:01 min.) explains the difference between heat and temperature, why a wind makes us feel colder, and what it's like to live as a mass of jiggling atoms.
Campbell, A., Jenden, J., Lloyd, E., Tierney, M., Donev, M., (2017, August 29). Thermal Energy . University of Calgary Energy Education
NASA. (n.d.) Beat the Heat!
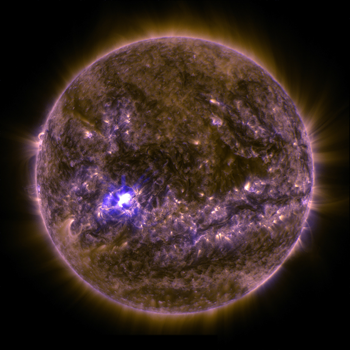
Perkins, S. (2016, September 30). Explainer: How heat moves . Science News for Students
University Corporation for Atmospheric Research, Centre for Science Education. (2018). Conduction .
Related Topics
- Random article
- Teaching guide
- Privacy & cookies

by Chris Woodford . Last updated: March 24, 2022.
Photo: Now that's what I call heat! The temperature of the SpaceX Falcon 9 space rocket exhaust you can see here is around 3000°C (5500°F) —hot enough to melt most everyday materials! Photo by Keegan Barber courtesy of NASA .
What is heat anyway?
Artwork: Hotter things have more heat energy than colder things. That's because the atoms or molecules move around faster in hot things (red, right) than they do in cold things (blue, left). This idea is called the kinetic theory.
What happens when something has no heat at all?
Photo: Ice may look cold but it's an awful lot hotter than absolute zero. Picture by Erich Regehr courtesy of US Fish & Wildlife Service .
What's the difference between heat and temperature?
Artwork: An iceberg is much colder than a cup of coffee but it contains more heat energy because it's so much bigger.
How can we measure temperature?
How does heat travel.
Animation: When you hold an iron bar in a fire, heat travels along the metal by conduction (red arrow). Why? Atoms at the hot end move more quickly as they absorb the fire's heat. They gradually pass their energy further along the bar, eventually warming the whole thing up.
Animation: How convection pumps heat into a saucepan. The pattern of warming, rising soup (red arrows) and falling, cooling soup (blue arrows) works like a conveyor that carries heat from the stove into the soup (orange arrows).
Picture: Infrared thermal images (sometimes called thermographs or thermograms) show that all objects give off some heat energy by radiation. In these two photos, you can see a rocket on a launch pad photographed with a normal camera (above) and an infrared thermal camera (below). The coldest parts are purple, blue, and black; the hottest areas are red, yellow, and white. Photo by R. Hurt, NASA/JPL-Caltech, courtesy of NASA.
Why do some things take longer to heat up than others?
Chart: Everyday materials have very different specific heat capacities. Metals (blue) have low specific heat capacities: they conduct heat well and store it badly, so they feel cold to the touch. Ceramic/mineral materials (orange) have higher specific heat capacitors: they don't conduct heat as well as metals, store it better, and feel slightly warmer when you touch them. Organic insulating materials (green), such as wood and leather, conduct heat very poorly and store it well, so they feel warm to the touch. With very high specific heat capacity, water (yellow) is in a class of its own.
Photo: The wooden spoon feels much warmer than the metal one, even though both are the same temperature. The metal spoon conducts heat more readily from your hand, and it's this that makes it feel colder.
Latent heat
Artwork: Normally things get hotter (their temperature rises) as you supply more heat energy. That doesn't happen at the points when things melt (change from solid to liquid) and vaporize (turn from liquid to gas). Instead, the energy you supply is used to change the state of matter . The energy doesn't vanish: it's stored as latent heat.
If you liked this article...
Find out more, on this website.
- Heat exchangers
- Heat insulation
For younger readers
- Heat by Ian Mahaney. Rosen, 2019. A 24-page, basic introduction for ages 8–10. It covers much the same scope as this article (where heat comes from, conduction, convection and radiation, measuring heat, heat capacity, and a few basic experiments).
- Investigating Heat by Sally M. Walker. Lerner Publications, 2017. This one is about 40 pages and also suitable for ages 8–10.
- Secrets of Heat and Cold by Andrew Solway. Encyclopedia Britannica, 2015. A question-and-answer-style introduction to the science of heat. Best for ages 8–10.
- Energy by Chris Woodford. Dorling Kindersley, 2007. My own book about energy includes a short section on heat energy. Suitable for ages 9–12.
For older readers
- Theory of Heat by James Clerk Maxwell. Longmans, 1871. Read Maxwell's statistical ideas about heat and the kinetic theory in his own words. The full text is available here in various electronic formats.
- Atoms under the Floorboards by Chris Woodford. Bloomsbury, 2015. One of my books for older readers. Chapter 13 is a simple introduction to heat and thermodynamics.
Text copyright © Chris Woodford 2009, 2022. All rights reserved. Full copyright notice and terms of use .
Rate this page
Tell your friends, cite this page, more to explore on our website....
- Get the book
- Send feedback
- Experiments
- Secret Science of Stuff
- Science ABCs
- Heat- Energy on the Move
- You are here:
- American Chemical Society
- Adventures in Chemistry
Heating a substance makes its atoms and molecules move faster. This happens whether the substance is a solid, a liquid, or a gas. It’s not easy to see it happen in a solid but let’s try it for a liquid and a gas. See if you can tell that heat makes molecules move!
Here's what to do:
- Place some ice in a large cup and add water to make ice water. After the water is cold, pour the ice water (without the ice) into a cup so that it is about ¾ full.
- Have your adult partner help you add hot water to another cup until it is ¾ full.
- At the same time, you and your adult partner should put one drop of yellow and one drop of blue food coloring on the surface of the hot and cold water.

What do you notice about the way the food coloring moves in the two cups?

What to expect
The food coloring in the hot water will move around and mix more and become more green than the food coloring in the cold water.
What's happening in there?
Heat is a form of energy. The heat energy from the water makes the water molecules in the hot water move faster than the water molecules in the cold water. The faster moving molecules in the hot water bump into the food coloring molecules more often and with more force and move them all around faster than the slower moving water molecules in the cold water.
What else could you try?
You could see if heating the molecules of a gas makes those molecules move faster. Let’s try it!
What you'll need:
- Hot tap water
- Cold tap water
- 3 plastic cups (wide enough for plastic bottle to fit in)
- 8 oz plastic bottle (from bottled water)
- Liquid dish detergent
Be sure to review the safety instructions on page 1 before proceeding.
- Add hot water to one cup and cold water to another cup until they are each about half-full.
- In a separate empty cup, make a detergent solution by mixing ½ teaspoon of liquid dish detergent with 1 tablespoon of water.
- Lower the open mouth of the bottle into the cup with detergent. Carefully tilt and lift the bottle out so that a detergent film covers the opening of the bottle.

- Slowly push the bottom of the bottle down into the hot water. What happens?

- Now push the bottom of the bottle into the cold water. What happens?

When the bottle is in hot water, a bubble will form on the top of the bottle. When the bottle is placed in cold water, the bubble will shrink.
When the bottle is placed in hot water, the heat energy from the water makes the molecules in the gas inside the bottle move faster and spread further apart. As they spread further apart, they push against the detergent film and form a bubble.
When the bottle is then placed in cold water, the gas molecules slow down and the bubble shrinks.
- Resources for Parents and Teachers
- Terms of Use
- Accessibility
Copyright © 2024 American Chemical Society

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
Margin Size
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

4.2: How Glaciers Work
- Last updated
- Save as PDF
- Page ID 25514

- Steven Earle
- Vancover Island University via BCCampus
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
There are two main types of glaciers. Continental glaciers (a.k.a. ice sheets) cover vast areas of land and only exist now in extreme polar regions, including Antarctica and Greenland (Figure 4.2.1). Alpine glaciers (a.k.a. valley glaciers) originate on mountains, mostly in temperate and polar regions, but even in tropical regions if the mountains are high enough. They are typically confined to valleys.

The Earth’s two great continental glaciers, on Antarctica and Greenland, comprise about 99% of all of the world’s glacial ice, and approximately 68% of all of the Earth’s fresh water. As is evident from Figure 4.2.2, the Antarctic ice sheet is vastly bigger than the Greenland ice sheet; it contains about 17 times as much ice. If the entire Antarctic ice sheet was to melt, sea level would rise by about 80 m and almost all of the Earth’s major cities would be submerged.
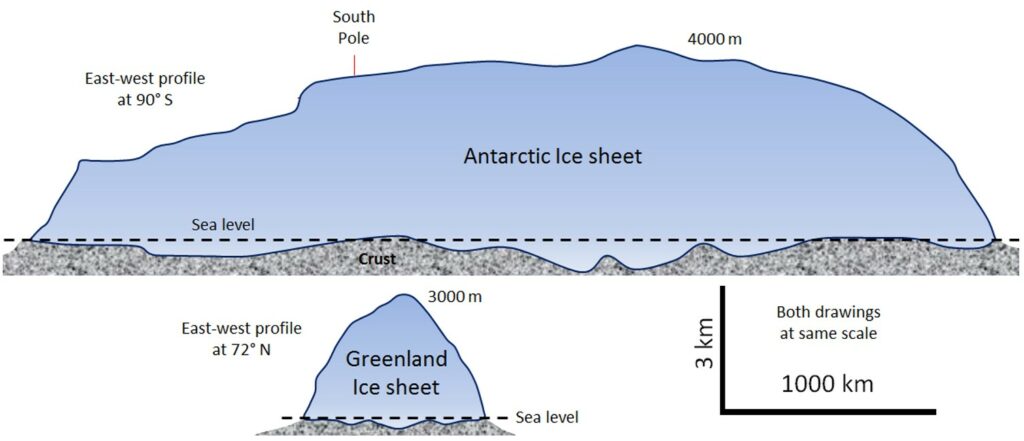
Continental glaciers do not flow “downhill” because the large areas that they cover are generally flat. Instead, ice flows from the region where it is thickest towards the edges where it is thinner. This is shown schematically on Figure 4.2.3. It means that in the central thickest parts the ice flows almost vertically down towards the base, while in the peripheral parts it flows outwards towards the margins. In continental glaciers like Antarctica and Greenland, the thickest parts (4000 and 3000 m respectively) are the areas where the rate of snowfall (and therefore of ice accumulation) are highest.

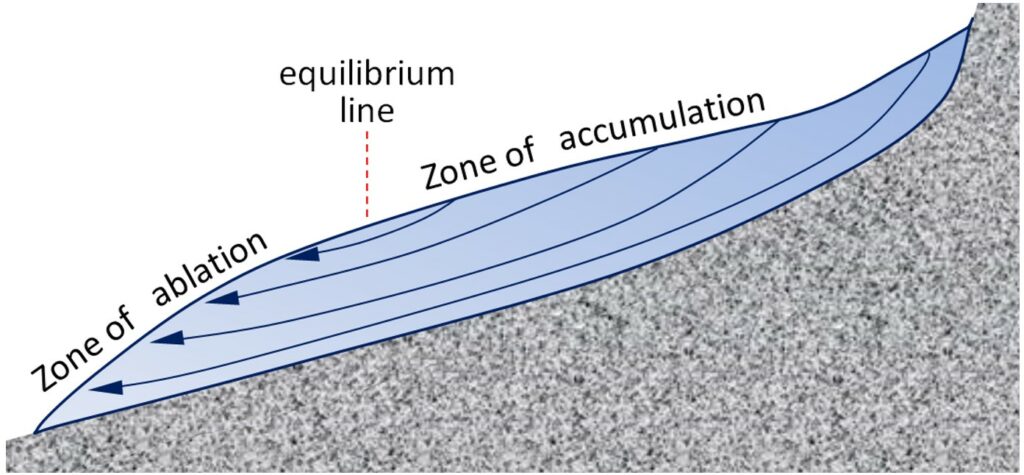
The flow of alpine glaciers is primarily controlled by the slope of the land beneath (Figure 4.2.4). In the zone of accumulation the rate of snowfall is greater than the rate of melting. In other words, not all of the snow that falls each winter melts during the following summer, and the ice surface is always covered with snow. In the zone of ablation more ice melts than accumulates as snow. The equilibrium line marks the boundary between the zones of accumulation and ablation.
The equilibrium line of the Overlord Glacier, near to Whistler BC, is shown in the photo on Figure 4.2.5. Below that line, in the zone of ablation, bare ice is exposed because last winter’s snow has all melted; above that line the ice is still mostly covered with snow from the last winter. The position of the equilibrium line changes from year to year as a function of the balance between snow accumulation in the winter and snow-melt during the summer. More winter snow and less summer melting obviously favours the advance of the equilibrium line (and of the glacier’s leading edge), but of these two variables it is the summer melt that matters most to a glacier’s budget. Cool summers promote glacial advance and warm summers promote glacial retreat.

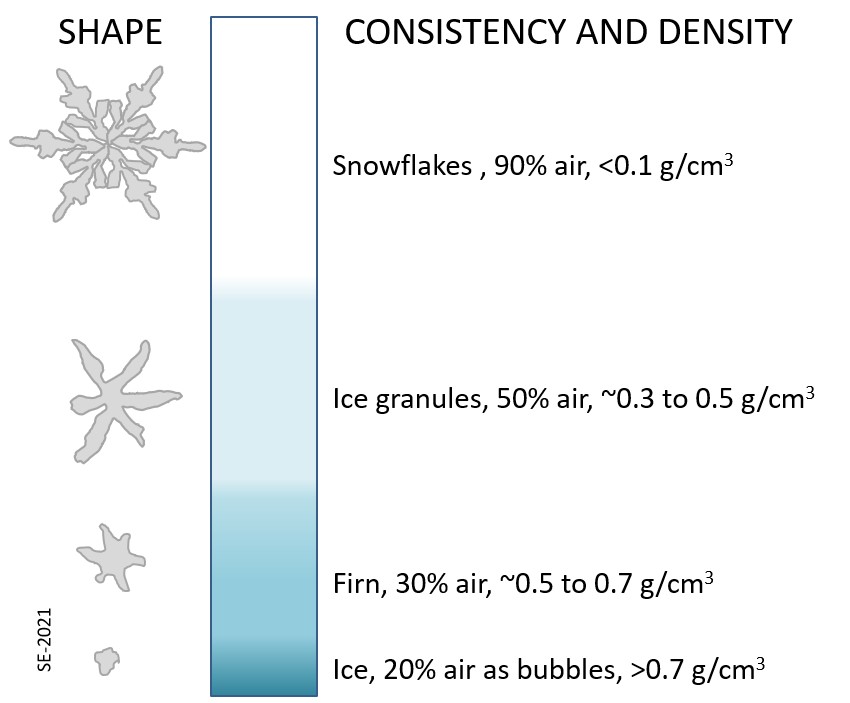
Above the equilibrium line of a glacier not all of the winter snow melts in the following summer, and thus snow gradually accumulates. The snow layer from each year is covered and compacted by subsequent snow and it gradually gets compressed and turned into firn within which the snowflakes lose their delicate shapes and become granules. With more compression the granules are pushed together and air is squeezed out. Eventually the granules are “welded” together to create glacial ice. Downward percolation of water, from melting taking place at surface, contributes to the process of ice formation (Figure 4.2.6).
Glaciers move because the surface of the ice is sloped. This generates a stress on the ice, which is proportional to the slope and the depth below the surface. As shown on Figure 4.2.7, the stresses are quite small near to the ice surface but much larger at depth, and also greater in areas where the ice surface is relatively steep. Ice will deform, meaning that it will behave in a plastic manner, at stress levels of around 100 kilopascals, and so it’s evident that, in this case, the upper 50 to 100 m of the ice (above the dashed red line) is not plastic (it is rigid) while the lower ice is plastic and will flow. The rigid layer will be thinner where the ice surface is steeper and thicker where it is flatter.
When the lower ice of a glacier flows it moves the upper ice along with it, so although it might seem from the stress patterns (red numbers and red arrows) shown on Figure 4.2.7 that the lower part should move the most, in fact while the lower part deforms (and flows) and the upper part doesn’t deform at all, the upper part moves the fastest because it is pushed along by the lower ice.

The plastic lower ice of a glacier can flow like a very viscous fluid, and it can therefore flow over irregularities in the base of the ice, and also around corners. But the upper rigid ice cannot flow in this way, and because it is being carried along by the lower ice it tends to crack where the lower ice has to flex. This leads to the development of crevasses in areas where the rate of flow of the plastic ice is changing. In the area shown on Figure 4.2.8, for example, the glacier is speeding up over the steep terrain, and the rigid surface ice has to crack to account for the change in velocity.

The base of a glacier can be cold (below the freezing point of water) or warm (above the freezing point). If it is warm there will likely be a film of water between the ice and the material underneath, and the ice will be able to slide over that surface. This is known as basal sliding (Figure 4.2.9, left). If the base is cold the ice will be frozen to the material underneath and it will be stuck—unable to slide along its base. In this case all of the movement of the ice will be by internal flow (Figure 4.2.9, right).
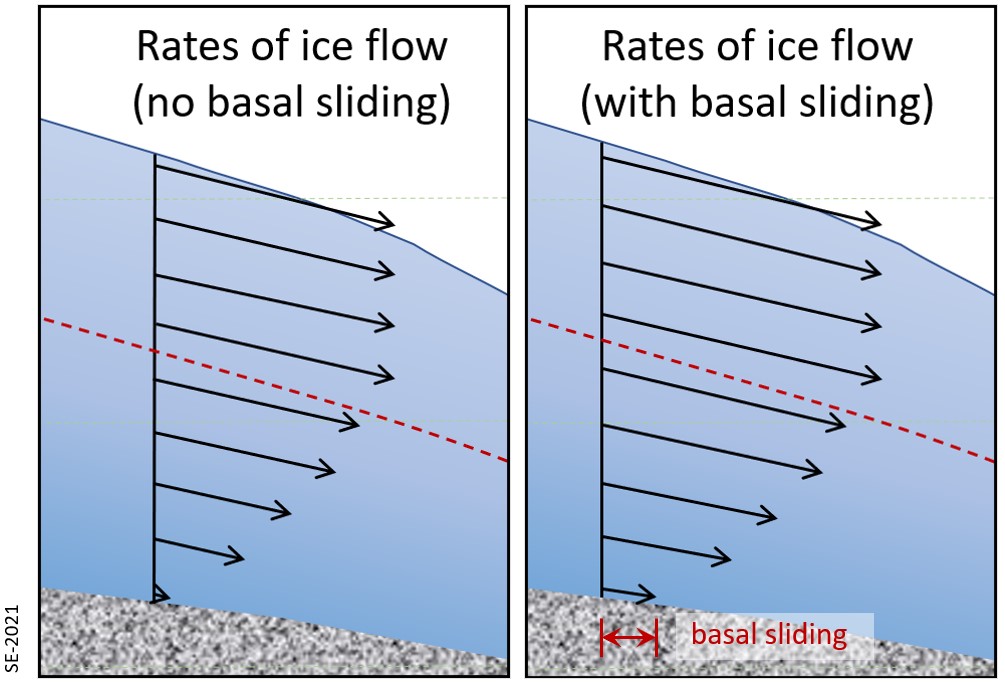
One of the factors that affects the temperature of the base of a glacier is the thickness of the ice. Ice is a good insulator. The slow transfer of heat from the Earth’s interior will provide enough heat to warm up the base if the ice is thick, but not enough if it is thin and that heat can escape. It is typical for the leading edge of an alpine glacier to be relatively thin (see Figure 4.2.7), and so it is common for that part to be frozen to its base while the rest of the glacier is still sliding. This is illustrated on Figure 4.2.10 for the Athabasca Glacier. Because the leading edge of the glacier is stuck to its frozen base, while the rest continues to slide, the ice coming from behind has pushed (or thrust) itself over top of the part that is stuck fast.

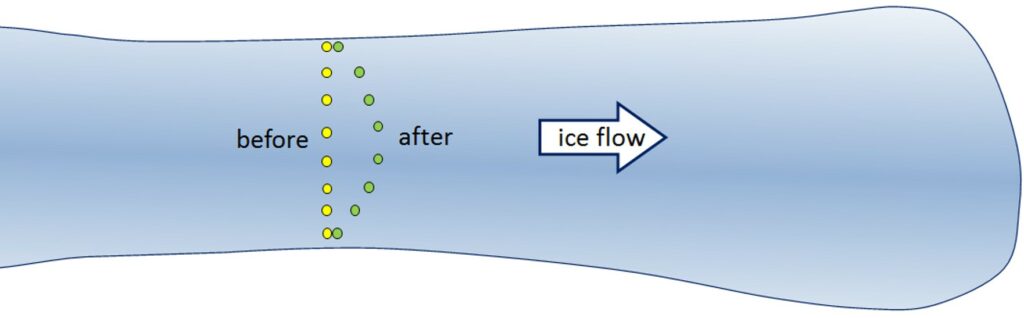
Just as the base of a glacier moves slower than the surface, the edges, which are more affected by friction along the sides, move slower than the middle. If we were to place a series of markers across an alpine glacier and come back a year later we would see that the ones in the middle have moved further forward than the ones near to the edges (Figure 4.2.11).
Glacial ice always moves downhill (or down from an area of thicker ice in the case of continental glaciers), in response to gravity, but the front edge of a glacier is almost always melting or else calving into water (shedding icebergs). Alpine glaciers can flow up over bumps in the terrain if the ice is thick enough. If the rate of forward motion of the glacier is faster than the rate of ablation (melting) the leading edge of the glacier will advance (move forward). If the rate of forward motion is about the same as the rate of ablation, the leading edge will remain stationary, and if the rate of forward motion is slower than the rate of ablation, the leading edge will retreat (move backward). Even if a glacier is retreating, the ice of the glacier will be moving forward.
Calving of icebergs is an important process for glaciers that terminate in lakes or the ocean. An example is shown on Figure 4.2.12.

Exercise 4.2 Moving Ice
Figure 4.2.13 represents a glacier onto the surface of which some markers (yellow dots) have been placed to determine the rate of ice motion over a one-year period. The ice is flowing from left to right.
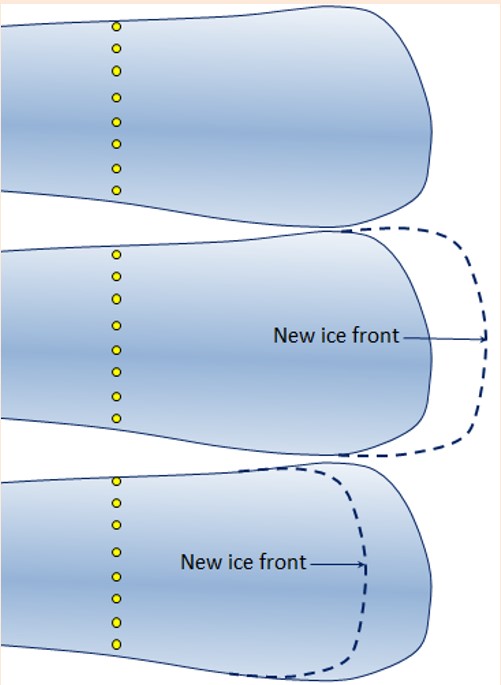
In the middle diagram the leading edge of the glacier has advanced. Draw in where the markers might have moved to.
In the lower diagram the leading edge of the glacier has retreated. Draw in where the markers might have moved to.
Exercise answers are provided Appendix 2 .
Media Attributions
- Figure 4.2.1 Photo by Ginny McLean, used with permission, CC BY 4.0
- Figure 4.2.2 Steven Earle, CC BY 4.0
- Figure 4.2.3 Steven Earle, CC BY 4.0
- Figure 4.2.4 Steven Earle, CC BY 4.0
- Figure 4.2.5 Modified by Steven Earle, CC BY 4.0 , from a photo by Isaac Earle, 2013. Used with permission.
- Figure 4.2.6 Steven Earle, CC BY 4.0
- Figure 4.2.7 Steven Earle, CC BY 4.0
- Figure 4.2.8 Photo by Isaac Earle, used with permission, CC BY 4.0
- Figure 4.2.9 Steven Earle, CC BY 4.0
- Figure 4.2.10 Steven Earle, CC BY 4.0
- Figure 4.2.11 Steven Earle, CC BY 4.0
- Figure 4.2.12 Photo by Isaac Earle, 2015, used with permission, CC BY 4.0
- Figure 4.2.13 Steven Earle, CC BY 4.0
Glaciers: Moving Rivers of Ice
A glacier is a huge mass of ice that moves slowly over land
Earth Science, Geology, Geography, Physical Geography
Loading ...

A glacier is a huge mass of ice that moves slowly over land. The term “ glacier ” comes from the French word glace (glah-SAY), which means ice . Glaciers are often called “ rivers of ice .” Glaciers fall into two groups: alpine glaciers and ice sheets . Alpine glaciers form on mountainsides and move downward through valleys . Sometimes, alpine glaciers create or deepen valleys by pushing dirt , soil , and other materials out of their way. Alpine glaciers are found in high mountains of every continent except Australia (although there are many in New Zealand). The Gorner Glacier in Switzerland and the Furtwangler Glacier in Tanzania are both typical alpine glaciers . Alpine glaciers are also called valley glaciers or mountain glaciers . Ice sheets , unlike alpine glaciers , are not limited to mountainous areas. They form broad domes and spread out from their centers in all directions. As ice sheets spread, they cover everything around them with a thick blanket of ice , including valleys , plains , and even entire mountains . The largest ice sheets , called continental glaciers , spread over vast areas. Today, continental glaciers cover most of Antarctica and the island of Greenland.
Massive ice sheets covered much of North America and Europe during the Pleistocene time period. This was the last glacial period , also known as the Ice Age . Ice sheets reached their greatest size about 18,000 years ago. As the ancient glaciers spread, they carved and changed the Earth’s surface, creating many of the landscapes that exist today. During the Pleistocene Ice Age , nearly one-third of the Earth’s land was covered by glaciers . Today, about one-tenth of the Earth’s land is covered by glacial ice . How Glaciers Form Glaciers begin forming in places where more snow piles up each year than melts. Soon after falling, the snow begins to compress , or become denser and tightly packed. It slowly changes from light, fluffy crystals to hard, round ice pellets . New snow falls and buries this granular snow. The hard snow becomes even more compressed . It becomes a dense , grainy ice called firn . The process of snow compacting into glacial firn is called firnification . As years go by, layers of firn build on top of each other. When the ice grows thick enough—about 50 meters (160 feet)—the firn grains fuse into a huge mass of solid ice . The glacier begins to move under its own weight. The glacier is so heavy and exerts so much pressure that the firn and snow melt without any increase in temperature . The melt water makes the bottom of the heavy glacier slicker and more able to spread across the landscape .
Pulled by gravity , an alpine glacier moves slowly down a valley . Some glaciers , called hanging glaciers , don't flow the entire length of a mountain . Avalanches and icefalls transfer glacial ice from hanging glaciers to a larger glacier beneath them, or directly to the valley below. An ice sheet spreads out from its center. The great mass of ice in a glacier behaves plastically , or like a liquid. It flows, oozes, and slides over uneven surfaces until it covers everything in its path. Different parts of a glacier move at different speeds. The flowing ice in the middle of the glacier moves faster than the base, which grinds slowly along its rocky bed. The different speeds at which the glacier moves causes tension to build within the brittle , upper part of the ice . The top of the glacier fractures , forming cracks called crevasses . Crevasses are in the top 50 meters (160 feet) of the glacier . Crevasses can be very dangerous for mountaineers . They can open quickly and be very deep. Moulins are another formation that carve into glaciers . A moulin is a deep, nearly-vertical pipeline in the glacier formed by melt water on top of the glacier falling through a crack in the ice . Moulins are often much deeper than crevasses , going all the way to the bottom of the glacier .
Most glaciers move very slowly—only a few centimeters a day. Some, though, can move 50 meters (160 feet) a day. These fast-moving rivers of ice are called galloping glaciers . Where a glacier meets the coast , it becomes a tidewater glacier . Its leading edge lifts and floats in the water , forming cliffs of ice that may be 60 meters (200 feet) high. Chunks of ice at the edge of the tide water glacier break away into the water —a process called calving . Calving is a violent process. It results in large waves and loud crashes. Floating chunks of glacial ice , broken off during calving , are called icebergs .
Glacial Features
Although glaciers move slowly, they are extremely powerful. Like huge bulldozers , they plow ahead year after year, crushing, grinding, and toppling almost everything in their paths. Forests , hills , and mountainsides are no match for glaciers .
Sometimes, glaciers form on volcanoes . When these volcanoes erupt, they are especially dangerous. They send floods of water , ice , and rocks over the land and into the atmosphere . Alpine glaciers begin to flow down hill from bowl-shaped mountain hollows called cirques . As the glaciers overflow the cirque , they move downward. They dig deep into the terrain , forming rugged, dramatic landscapes . As they move, glaciers erode or wear away the land beneath and around them. Glaciers carry great amounts of soil , rock , and clay . Some of the boulders they carry are as big as houses.
Rocks carried hundreds and even thousands of kilometers by glaciers are called glacial erratics . Glacial erratics differ significantly from the landscape in which they were deposited . The Big Rock , for instance, is a 15,000-ton quartzite boulder near Okotoks, Alberta, Canada. The Big Rock was deposited from what is now northern Alberta, about 1,640 kilometers (500 miles) away, during the last ice age . Embedded , or stuck, in a glacier ’s base, these large rocks grind against the ground like the prongs of a rake . They dig long grooves, called striations , in the surface of the Earth. Geologists can tell in what direction an ancient glacier moved by studying striations left in rock . Glaciers eventually deposit their loads of rock , dirt , and gravel . These materials are called moraine . Piles of moraine dumped at a glacier ’s end, or snout , are called terminal moraines .
Lateral moraine forms along the side of a glacier . Medial moraine appears as dark lines near the center of the glacier . Supraglacial moraine appears on the surface of the glacier — dirt , dust , leaves, and anything else that falls onto a glacier and sticks. Ogives are frozen “waves,” or ridges, on the surface of a glacier .
When glaciers began their final retreat 10,000 years ago, they left behind many landscape features, such as lakes , valleys , and mountains . Many hollowed-out areas carved by glaciers became lakes . Bowl-shaped cirques , where most alpine glaciers form, became mountain lakes . These alpine lakes are called tarns . Glaciers can also create lakes by leaving depressions in the earth. The Finger Lakes in the western part of the U.S. state of New York were excavated during the last Ice Age . The lakes were once stream valleys . Along the streams , the glacier scooped out troughs that now contain deep lakes .
Glacial retreat created other features of the landscape . Materials deposited by a glacier as it retreats are called ground moraines . The jumble of rock , gravel , and dirt making up ground moraines is called till . Much of the fertile soil in the Great Plains of North America was formed from layers of till left by ancient ice sheets .
Glacial valleys exist on almost every continent . These valleys are scooped out as a glacier scrapes through them. There are no glaciers in Australia, but Mount Kosciuszko s till has glacial valleys from the last Ice Age . Distinctive mountain formations called aretes and horns are the result of glacial activity. An arête is a sharp ridge of rock that forms when two glaciers collide . Each glacier erodes a glacial valley on either side of the arête. Glacier National Park in the U.S. state of Montana is filled with deep glacial valleys and sharp arêtes. An arête where three or more glaciers meet to form a peak is called a horn . These tall, singular landforms are also called pyramidal peaks . The Matterhorn in Switzerland and Italy (and its copy in Disneyland, California) is a glacial horn . Roche moutonnee is a smooth, rounded rock formation created as a glacier crushes and rearranges rocks in its path. Roche moutonnee is visible in many hilly areas as outcroppings of flat rock . In contrast to alpine glaciers , ice sheets do not create landscape features as they spread. They tend to smooth out the land beneath them.
People and Glaciers
Glaciers provide people with many useful resources. Glacial till provides fertile soil for growing crops . Deposits of sand and gravel are used to make concrete and asphalt .
The most important resource provided by glaciers is freshwater . Many rivers are fed by the melting ice of glaciers . The Gangotri Glacier , one of the largest glaciers in the Himalayan Mountains , is the source of the River "> Ganges River . The Ganges is the most important source of fresh water and electricity in India and Bangladesh. ( Electri city is created by dams and hydroelectric power plants along the Ganges.) Some companies link glacial water to clean, fresh taste. Because water has been trapped in the glacier for so long, many people believe it has not been exposed to pollutants that liquid water is exposed to. Glaciers dug basins for most of the world’s lakes and carved much of the Earth’s most spectacular mountain scenery. The dramatic , diverse landscape of Yosemite Valley , California, was sculpted entirely by glaciers during the last Ice Age . Threats to Glaciers The processes that remove snow, ice , and moraine from a glacier or ice sheet are called ablation . Ablation includes melting, evaporation, erosion, and calving . Glaciers melt when ice melts more quickly than firn can accumulate . Earth’s average temperature has been increasing dramatically for more than a century . Glaciers are important indicators of global warming and climate change in several ways. Melting ice sheets contribute to rising sea levels . As ice sheets in Antarctica and Greenland melt, they raise the level of the ocean . Tons of fresh water are added to the ocean every day. In March 2009, a 160-square-mile piece of the Wilkins Ice Shelf broke off of the Antarctic Peninsula. Large icebergs created by such an event create hazards for shipping . Large additions of fresh water also change the ocean ecosystem . Organisms, such as many types of corals , depend on salt water for survival. Some corals may not be able to adjust to a more fresh water habitat. The loss of glacial ice also reduces the amount of fresh water available for plants and animals that need fresh water to survive. Glaciers near the Equator , such as those on the tropical island of Papua or in South America, are especially at risk. The residents below Chacaltaya Glacier in Bolivia, for instance, depended on the glacier for almost all of their fresh water and electri city . Chacaltaya Glacier provided these resources to La Paz, Bolivia’s largest city . Chacaltaya Glacier was also the world’s highest ski resort . In 2009, Chacaltaya Glacier melted entirely. A few glaciers may actually be benefiting from global warming . Although winter temperatures are rising, so is the amount of snowfall in areas like Pakistan’s Upper Indus River Basin. Glaciers are growing quickly there. Less precipitation also affects some glaciers . In 1912, the glaciers on Tanzania’s Mount Kilimanjaro covered 12 square kilometers (4.6 square miles). In 2009, Kilimanjaro’s alpine glaciers had shrunk to two square kilometers (0.8 square miles). This reduction is the result of few heavy snowfalls .
Why So Blue? Some glaciers and icebergs are blue, for the same reason water is blue. The chemical bond between oxygen and hydrogen in water absorbs light in the red end of the visible light spectrum. Blue glaciers and icebergs are not blue for the same reason the sky is blue. The sky is blue due to atmospheric scattering of light (Raleigh scattering), a different phenomenon.
Icefall Glaciers are called "rivers of ice." Just like rivers, glaciers have fall lines where the bed of the glacier gets narrow or descends rapidly. Ice flows down the icefall just like water falls down a waterfall. The Khumbu Icefall is one of the most difficult terrains on Mount Everest.
Paleoclimatology Paleoclimatology is the study of the Earth's atmosphere in prehistoric times. Paleoclimatology depends on ice and bubbles in glaciers and ice sheets. Scientists extract long tubes of ice, called ice cores, from thick ice sheets, usually in the Antarctic. Ice cores are layered, with the deepest ice having the oldest information. Wide bands indicate a heavy snowfall. Darkly colored bands indicate smoke or other chemicals in the atmosphere. Ice cores can measure the state of the atmosphere as far back as 80,000 years. For instance, cores from ice sheets from the year 1883 contained chemicals from the massive eruption of Krakatoa, a volcanic island in Indonesia. Ice cores showed those chemicals drifted from the South Pacific to Antarctica and Greenland and stayed in the atmosphere for many years afterward.
Third Pole Siachen Glacier, a huge glacier in the Himalayan Mountains, is sometimes called the Third Pole. Siachen Glacier is the worlds highest area of conflict. Although India controls Siachen, both India and Pakistan claim the area as part of their country. Siachen Glacier is the site of the worlds highest helicopter landing pad, which India built for military and emergency use.
Media Credits
The audio, illustrations, photos, and videos are credited beneath the media asset, except for promotional images, which generally link to another page that contains the media credit. The Rights Holder for media is the person or group credited.
Illustrators
Educator reviewer, last updated.
October 19, 2023
User Permissions
For information on user permissions, please read our Terms of Service. If you have questions about how to cite anything on our website in your project or classroom presentation, please contact your teacher. They will best know the preferred format. When you reach out to them, you will need the page title, URL, and the date you accessed the resource.
If a media asset is downloadable, a download button appears in the corner of the media viewer. If no button appears, you cannot download or save the media.
Text on this page is printable and can be used according to our Terms of Service .
Interactives
Any interactives on this page can only be played while you are visiting our website. You cannot download interactives.
Related Resources
Ice floes, ice sheets and the sea

> There are large areas in the polar regions where water occurs predominantly in its frozen state. It either falls as snow to contribute to the growth of ice sheets and glaciers, or it drifts on the sea as ice floes. In both cases the fate of the ice depends largely on the ocean and its currents. The water masses can provide protection or accelerate melting, depending on the path that heat follows.
Sea-ice nurseries

Location matters

When spring comes
What drives the ocean currents.
- The ocean currents transport an immense amount of heat energy and distribute it around the world.
- Variously warm air currents and water currents regulate the Earth’s water cycle through the evaporation of seawater and the absorption or release of heat at the sea surface, depending on whether the overlying atmosphere is colder or warmer than the water.
At right angles to the wind

Density changes – ascending or descending?
A question of salinity.

Overturning in the wild Southern Ocean

Overturning in the Arctic Ocean

- cold, low-salinity surface water that is driven by the wind and distributed into the central Arctic;
- cold, high-salinity water that sinks to intermediate depths and spreads out there; and
- very salty, heavy water masses that either flow directly through the Norwegian Sea back to the Atlantic or take the longer route as Arctic Bottom Water through the Arctic Basin and the Fram Strait.

A protective layer for the sea ice
The surface layer.

The halocline

The Atlantic Water
The arctic deep water.
Tabular iceberg A freshly calved tabular iceberg has a flat, level surface and nearly vertical flanks. In the Antarctic these icebergs can be up to 160 kilometres long and tens of kilometres wide. As a rule, they are 200 to 400 metres thick and rise 30 to 50 metres above the water surface. Similarly shaped icebergs in the Arctic are generally much smaller.

Continental-scale ice sheets

From snow to ice in three steps
1. snow compaction, 2. firn formation.

3. Ice formation

Why does ice flow?
Ice streams, 1. topographic constraint, 2. topographic steps, 3. unevenness of the bedrock, 4. break-off of icebergs, 5. deformable sediments beneath the ice sheet.
- Firstly, the sediments, as a covering layer, smooth out existing irregularities in the subsurface and thus reduce its unevenness.
- Secondly, a sediment layer saturated with melt water creates an optimal sliding surface. Anyone who has slipped in the mud as a child knows that this sliding effect is not experienced on a dry, paved or asphalted surface.
- And thirdly, sediment deposits are easily deformed under the weight of the ice. Under some circumstances, they may even slip themselves and thus clear the way or accelerate the ice flow.
6. Geothermal heat

7. Meltwater lakes and rivers
How ice shelves retard glacial flow.
Introduction to electromagnetic waves
Basic properties of waves: amplitude, wavelength, and frequency, example: calculating the wavelength of a light wave, the electromagnetic spectrum, quantization of energy and the dual nature of light, example: calculating the energy of a photon, attributions.
- “ Electromagnetic Radiation ” from UC Davis ChemWiki, CC BY-NC-SA 3.0
Additional References
Want to join the conversation.
- Upvote Button navigates to signup page
- Downvote Button navigates to signup page
- Flag Button navigates to signup page

- svg]:stroke-accent-900">
How to make crystal-clear ice cubes
By Hugh Neill
Posted on Aug 12, 2022 12:34 PM EDT
4 minute read
The sound of crackling ice is synonymous with cool refreshment. On a literal level, it’s the sound of structural damage creating smaller pieces of ice with more surface area that will melt faster. That’s not necessarily a problem, but sometimes you want ice that keeps your drink colder longer, retains its shape, and dilutes slowly. For that you need to make perfectly clear ice.
Most ice you make at home or purchase from a store is at least a bit cloudy. While this cloudiness is often attributed to vague “impurities,” the cleanliness of your starting water has little to do with it. Of course, you should always start with clean water, but even an ice tray full of filtered, boiled water will still produce cubes with cloudy centers.
Why does my ice look cloudy?
When you fill an ice cube tray and pop it in the freezer, it sits in an uninsulated container that’s exposed to cold air from all directions, and begins to freeze from the outside in. If you look at an ice cube halfway through the freezing process, when its core is still liquid, it will likely look crystal-clear. “Crystal-clear” is an apt term because at this stage, the crystal structure of the ice will still be consistent and strong in the outer layers.
Within those outer layers of your half-frozen cubes, there will be a bubble of air just waiting to cloud up your ice. When the expanding ice crystal matrix closes in on that bubble, the trapped air will disrupt the clear crystal formation and create that fuzzy white web we’re all so used to. In more scientific terms, the trapped air has a lower thermal load than water and expands differently when the temperature changes. This causes differential expansion rates between the inner and outer layers of your ice when it touches liquid, resulting in internal fissures—the crackling you hear in your glass.
How to make clear ice
If you want beautiful ice and all the benefits that come with it, there are three ways to make it at home. The first method is the cheapest but most labor-intensive, the second method is faster but more expensive, and the third is just buying a big machine to do it for you. All three rely on controlling the direction and speed of ice crystal formation.
Method 1: a cooler and a serrated knife
The most important factor in making clear ice is directional freezing, by which I mean ensuring that the ice forms in one direction rather than from all sides. In practice, this means using a container that’s insulated everywhere but on top, such as a cooler. Find a cooler that fits in your freezer and fill it with clean water, leaving about an inch empty on top to allow room for expansion. Err on the side of caution with more empty space than you think you need when you first try it, as the alternative is cracking your cooler. Pop it in the freezer and wait 24 hours. The water will freeze from the top down, and the trapped air will all get pushed to the bottom.
When time’s up, remove the cooler and invert it onto a surface you’re not afraid to get wet. There will likely still be a layer of liquid water and trapped air at the very bottom, and all that’s going to come pouring out. This also means the air won’t have had a chance to cloud your ice, and you will have a nice, clear slab.
[Related: The scientifically best way to pack a cooler ]
Now, cut the ice to the desired size. Using a sturdy serrated knife, score grooves into the ice, about 1 centimeter deep, and then tap the back of the blade with a hammer to break through the rest of the way. Repeat this process and you’ll have perfectly clear, solid ice cubes of custom size.
Method 2: an insulated ice tray
Get a directional freezing ice mold for cubes (or spheres if you’re feeling fancy) . It will be insulated on all sides but the top, and the ice cube tray itself should have a small hole in the bottom of the ice chamber for the air to pass through. There will be an extra chamber beneath the cube mold for the air and cloudy ice to get trapped in, so everything that comes out of the mold itself will be clear. Like the cooler method, this works best if you take it out of the freezer before it has frozen all the way to the bottom, ensuring cloudy ice hasn’t had a chance to form.
Method 3: buy a big machine
A decent countertop clear ice machine will run you about $150 to $250, but if your demand for ice is high enough that you’re routinely buying bags of it, it may be a worthwhile investment. These machines will result in smaller pieces of ice, but will save you time and effort—as machines are supposed to. Most home clear ice machines work by dripping a constant flow of recirculated water over an extremely chilled metal lattice with an open front, so the ice crystals form back to front, and there’s never enough water freezing at once for air to get trapped. The Sentern portable countertop clear ice maker and the NewAir ClearIce40 both work on this principle and are well-reviewed, but you’ll need to immediately use or freeze the ice they make, or—as you may have guessed—your pristine cubes will melt.
Latest in Life Skills
How often should you vacuum it depends. how often should you vacuum it depends..
By Debbie Wolfe
Is the Y cut the best way to slice a sandwich? Is the Y cut the best way to slice a sandwich?
By Justin Pot

Suggested Searches
- Climate Change
- Expedition 64
- Mars perseverance
- SpaceX Crew-2
- International Space Station
- View All Topics A-Z
Humans in Space
Earth & climate, the solar system, the universe, aeronautics, learning resources, news & events.

NASA Invites Public to Share Excitement of NOAA GOES-U Launch

NASA’s ELaNa 43 Prepares for Firefly Aerospace Launch
Why scientists are intrigued by air in nasa’s mars sample tubes.
- Search All NASA Missions
- A to Z List of Missions
- Upcoming Launches and Landings
- Spaceships and Rockets
- Communicating with Missions
- James Webb Space Telescope
- Hubble Space Telescope
- Why Go to Space
- Commercial Space
- Destinations
- Living in Space
- Explore Earth Science
- Earth, Our Planet
- Earth Science in Action
- Earth Multimedia
- Earth Science Researchers
- Pluto & Dwarf Planets
- Asteroids, Comets & Meteors
- The Kuiper Belt
- The Oort Cloud
- Skywatching
- The Search for Life in the Universe
- Black Holes
- The Big Bang
- Dark Energy & Dark Matter
- Earth Science
- Planetary Science
- Astrophysics & Space Science
- The Sun & Heliophysics
- Biological & Physical Sciences
- Lunar Science
- Citizen Science
- Astromaterials
- Aeronautics Research
- Human Space Travel Research
- Science in the Air
- NASA Aircraft
- Flight Innovation
- Supersonic Flight
- Air Traffic Solutions
- Green Aviation Tech
- Drones & You
- Technology Transfer & Spinoffs
- Space Travel Technology
- Technology Living in Space
- Manufacturing and Materials
- Science Instruments
- For Kids and Students
- For Educators
- For Colleges and Universities
- For Professionals
- Science for Everyone
- Requests for Exhibits, Artifacts, or Speakers
- STEM Engagement at NASA
- NASA's Impacts
- Centers and Facilities
- Directorates
- Organizations
- People of NASA
- Internships
- Our History
- Doing Business with NASA
- Get Involved
- Aeronáutica
- Ciencias Terrestres
- Sistema Solar
- All NASA News
- Video Series on NASA+
- Newsletters
- Social Media
- Media Resources
- Upcoming Launches & Landings
- Virtual Events
- Sounds and Ringtones
- Interactives
- STEM Multimedia

Amendment 22: Heliophysics Flight Opportunities in Research and Technology Final Text and Due Date

Mercury Resources

Former Astronaut Charles M. Duke, Jr.

Lakita Lowe: Leading Space Commercialization Innovations and Fostering STEM Engagement

Former Astronaut Russell L. “Rusty” Schweickart
The Ocean and Climate Change

NASA Satellites Find Snow Didn’t Offset Southwest US Groundwater Loss

NASA-Led Mission to Map Air Pollution Over Both U.S. Coasts

Perseverance Finds Popcorn on Planet Mars

Hubble Captures Infant Stars Transforming a Nebula

First of Its Kind Detection Made in Striking New Webb Image

NASA Releases Hubble Image Taken in New Pointing Mode

Hypersonic Technology Project

NASA Engineer Honored as Girl Scouts ‘Woman of Distinction’

NASA, MagniX Altitude Tests Lay Groundwork for Hybrid Electric Planes

Augmented Reality Speeds Spacecraft Construction at NASA Goddard

Giant Batteries Deliver Renewable Energy When It’s Needed
Slow Your Student’s ‘Summer Slide’ and Beat Boredom With NASA STEM

Next Generation NASA Technologies Tested in Flight

Astronauta de la NASA Frank Rubio

Diez maneras en que los estudiantes pueden prepararse para ser astronautas

Astronauta de la NASA Marcos Berríos
Nasa mission flies over arctic to study sea ice melt causes.
Erica McNamee
It’s not just rising air and water temperatures influencing the decades-long decline of Arctic sea ice. Clouds, aerosols, even the bumps and dips on the ice itself can play a role. To explore how these factors interact and impact sea ice melting, NASA is flying two aircraft equipped with scientific instruments over the Arctic Ocean north of Greenland this summer. The first flights of the field campaign, called ARCSIX (Arctic Radiation Cloud Aerosol Surface Interaction Experiment), successfully began taking measurements on May 28.

“The ARCSIX mission aims to measure the evolution of the sea ice pack over the course of an entire summer,” said Patrick Taylor, deputy science lead with the campaign from NASA’s Langley Research Center in Hampton, Virginia. “There are many different factors that influence the sea ice. We’re measuring them to determine which were most important to melting ice this summer.”
On a completely clear day over smooth sea ice, most sunlight would reflect back into the atmosphere, which is one way that sea ice cools the planet. But when the ice has ridges or darker melt ponds — or is dotted with pollutants — it can change the equation, increasing the amount of ice melt. In the atmosphere, cloudy conditions and drifting aerosols also impact the rate of melt.
“An important goal of ARCSIX is to better understand the surface radiation budget — the energy interacting with the ice and the atmosphere,” said Rachel Tilling, a campaign scientist from NASA’s Goddard Space Flight Center in Greenbelt, Maryland.
About 75 scientists, instrument operators, and flight crew are participating in ARCSIX’s two segments based out of Pituffik Space Base in northwest Greenland. The first three-week deployment, in May and June of this year, is timed to document the start of the ice melt season. The second deployment will occur in July and August to monitor late summer conditions and the start of the freeze-up period.
“Scientists from three key disciplines came together for ARCSIX: sea ice surface researchers, aerosol researchers, and cloud researchers,” Tilling said. “Each of us has been working to understand the radiation budget in our specific area, but we’ve brought all three areas together for this campaign.”
Two aircraft will fly over the Arctic during each deployment. NASA’s P-3 Orion aircraft from the agency’s Wallops Flight Facility in Virginia, will fly below the clouds at times to document the surface properties of the ice and the amount of energy radiating off it. The team will also fly the aircraft through the clouds to sample aerosol particles, cloud optical properties, chemistry, and other atmospheric components.
A Gulfstream III aircraft, managed by NASA Langley, will fly higher in the atmosphere to observe properties of the tops of the clouds, take profiles of the atmosphere above the ice, and add a perspective similar to that of orbiting satellites.
The teams will also compare airborne data with satellite data. Satellite instruments like the Multi-angle Imaging Spectroradiometer and the Moderate Resolution Imaging Spectroradiometer will provide additional information about clouds and aerosol particles, while the Ice, Cloud, and land Elevation Satellite 2 will provide insights into the ice topography below both satellites and aircraft.
The aircraft will fly coordinated routes to take measurements of the atmosphere above ice in three-dimensional space, said Sebastian Schmidt, the mission’s science lead with the University of Colorado Boulder.
“The area off the northern coast of Greenland can be considered the last bastion of multi-year sea ice, as the Arctic transitions to a seasonally ice-free ocean,” Schmidt said. “By observing here, we will gain insight into cloud-aerosol-sea ice-interaction processes of the ‘old’ and ‘new’ Arctic — all while improving satellite-based remote sensing by comparing what we’re seeing with the airborne and satellite instruments.”
By Kate Ramsayer
NASA’s Goddard Space Flight Center, Greenbelt, Md.
Related Terms
- Airborne Science
- Goddard Space Flight Center
- Ice & Glaciers
- Langley Research Center
- Wallops Flight Facility
Explore More

Antarctic Sea Ice Near Historic Lows; Arctic Ice Continues Decline

Arctic Sea Ice 6th Lowest on Record; Antarctic Sees Record Low Growth
Arctic sea ice likely reached its annual minimum extent on September 19, 2023, making it…

NASA Ice Scientists Take Flight from Greenland to Study Melting Arctic Ice
Does ice always travel in one direction?
Add your answer:
How did one direction travel to radio stations to promote what makes you beautiful?
Who is baby lux that's always around one direction.
Baby Lux is Lou's daughter . Lou is One Direction's stylist .
What was one direction original name?
not sure what you mean they have always been called one direction the only name change I can think that you may be referring to is zayn his real name is zain but he likes zayn better
Where does One Direction live?
i love one direction i always scream when ever i see them there are in my life if one direction what to see me i live in ma
Why is it helpful to have impulses move only in one direction in a neuron-?
Nerve impulses travel one direction because of the action potential which is created because of Na+ and when K+ returns to normal.
Does ice travel in one direction?
Why does blood always travel in a single direction.
Because of the one-way valves.
What does naill from one direction always travels with?
Naill always travels with the one direction members
Does electricity travel you One Direction?
no because lots of people use electricity around the world so it doesn't travel one direction
What direction can you travel from the north pole?
One step in any direction will take you south.
What type of current will a circuit that involves a battery and electrons traveling in one direction have?
a battery always produces a direct current.the electrons always travel from the negetiove to the positive terminal.But the direction of the current is the opposite that is from the positive to the negetive terminal.
Do Laser beams have one wavelength and travel in One Direction?
Who out of one direction eats ice cream with a fork, does sound travel in more than one direction, do tornadoes aways move in the same direction.
No. It is a common misconception that tornadoes always travel northeast. In truth, while t northeast is the most common direction for U.S. tornadoes, they can travel in any direction. In one famous case in 1997 an extremely powerful and deadly F5 tornado traveled southwest. The town of Jarrell, Texas was completely devastated.
The speed and direction a rocket must travel to break away from a planet's gravitational pull is called?
To escape the gravitation pull of an object you must travel at or in excess of the escape velocity. The direction of the escape velocity is always radially outward from the center of the object.
What do the boys from one direction like to do?
They like to sing travel and draw
Top Categories


- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
Margin Size
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

12.1: Traveling Waves
- Last updated
- Save as PDF
- Page ID 22274

- Julio Gea-Banacloche
- University of Arkansas via University of Arkansas Libraries
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
In our study of mechanics we have so far dealt with particle-like objects (objects that have only translational energy), and extended, rigid objects, which may also have rotational energy. We have, however, implicitly assumed that all the objects we studied had some internal structure, or were to some extent deformable, whenever we allowed for the possibility of their storing other forms of energy, such as chemical or thermal.
This chapter deals with a very common type of organized (as opposed to incoherent) motion exhibited by extended elastic objects, namely, wave motion . (Often, the “object” in which the wave motion takes place is called a “medium.”) Waves can be “traveling” or “standing,” and we will start with the traveling kind, since they are the ones that most clearly exhibit the characteristics typically associated with wave motion.
A traveling wave in a medium is a disturbance of the medium that propagates through it, in a definite direction and with a definite velocity. By a “disturbance” we typically mean a displacement of the parts that make up the medium, away from their rest or equilibrium position. The idea here is to regard each part of an elastic medium as, potentially, an oscillator, which couples to the neighboring parts by pushing or pulling on them (for an example of how to model this mathematically, see Advanced Topic 12.6 at the end of this chapter). When the traveling wave reaches a particular location in the medium, it sets that part of the medium in motion, by giving it some energy and momentum, which it then passes on to a neighboring part, and so on down the line.
You can see an example of how this works in a slinky. Start by stretching the slinky somewhat, then grab a few coils, bunch them up at one end, and release them. You should see a “compression pulse” traveling down the slinky, with very little distortion; you may even be able to see it being reflected at the other end, and coming back, before all its energy is dissipated away.
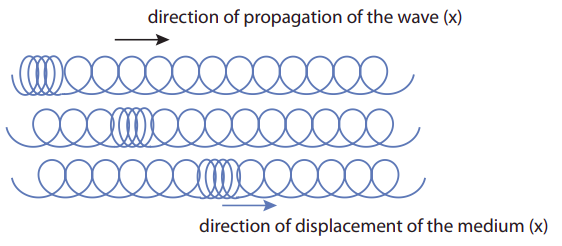
The compression pulse in the slinky in Figure \(\PageIndex{1}\) is an example of what is called a longitudinal wave , because the displacement of the parts that make up the medium (the rings, in this case) takes place along the same spatial dimension along which the wave travels (the horizontal direction, in the figure). The most important examples of longitudinal waves are sound waves , which work a bit like the longitudinal waves on the slinky: a region of air (or some other medium) is compressed, and as it expands it pushes on a neighboring region, causing it to compress, and passing the disturbance along. In the process, regions of rarefaction (where the density drops below its average value) are typically produced, alongside the regions of compression (increased density).
The opposite of a longitudinal wave is a transverse wave , in which the displacement of the medium’s parts takes place in a direction perpendicular to the wave’s direction of travel. It is actually also relatively easy to produce a transverse wave on a slinky: again, just stretch it somewhat and give one end a vigorous shake up and down. It is, however, a little hard to draw the resulting pulse on a long spring with all the coils, so in Figure \(\PageIndex{2}\) below I have instead drawn a transverse wave pulse on a string , which you can produce in the same way. (Strings have other advantages: they are also easier to describe mathematically, and they are very relevant, particularly to the production of musical sounds.)
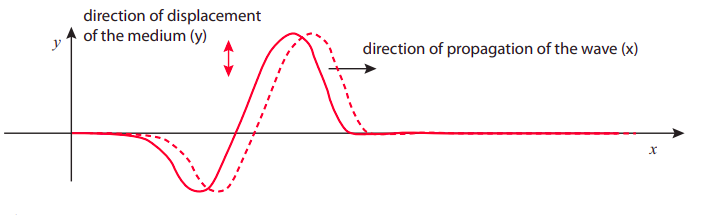
Perhaps the most important (and remarkable) property of wave motion is that it can carry energy and momentum over relatively long distances without an equivalent transport of matter . Again, think of the slinky: the “pulse” can travel through the slinky’s entire length, carrying momentum and energy with it, but each individual ring does not move very far away from its equilibrium position. Ideally, after the pulse has passed through a particular location in the medium, the corresponding part of the medium returns to its equilibrium position and does not move any more: all the energy and momentum it momentarily acquired is passed forward. The same is (ideally) true for the transverse wave on the string in Figure \(\PageIndex{2}\).
Since this is meant to be a very elementary introduction to waves, I will consider only this case of “ideal” (technically known as “linear and dispersion-free”) wave propagation, in which the speed of the wave does not depend on the shape or size of the disturbance. In that case, the disturbance retains its “shape” as it travels, as I have tried to illustrate in figures \(\PageIndex{1}\) and \(\PageIndex{2}\).
The "Wave Shape" Function- Displacement and Velocity of the Medium
In a slinky, what I have been calling the “parts” of the medium are very clearly seen (they are, naturally, the individual rings); in a “homogeneous” medium (one with no visible parts), the way to describe the wave is to break up the medium, in your mind, into infinitely many small parts or “particles” (as we have been doing for extended systems all semester), and write down equations that tell us how each part moves. Physically, you should think of each of these “particles” as being large enough to contain many molecules, but small enough that its position in the medium may be represented by a mathematical point.
The standard way to label each “particle” of the medium is by the position vector of its equilibrium position (the place where the particle sits at rest in the absence of a wave). In the presence of the wave, the particle that was initially at rest at the point \(\vec{r}\) will undergo a displacement that I am going to represent by the vector \(\vec{\xi}\) (where \(\xi\) is the Greek letter “xi”). This displacement will in general be a function of time, and it may also be different for different particles, so it will also be a function of \(\vec{r}\), the equilibrium position of the particle we are considering. The particle’s position under the influence of the wave becomes then
\[ \vec{r}+\vec{\xi}(\vec{r}, t) \label{eq:12.1} .\]
This is very general, and it can be given a simpler form for simple cases. For instance, for a transverse wave on a string, we can label each part of the string at rest by its \(x\) coordinate, and then take the displacement to lie along the \(y\) axis; the position vector, then, could be written in component form as \( (x, \xi(x, t), 0) \). Similarly, we can consider a “plane” sound wave as a longitudinal wave traveling in the \(x\) direction, where the density of the medium is independent of \(y\) and \(z\) (that is, it is constant on planes perpendicular to the direction of propagation). In that case, the equilibrium coordinate \(x\) can be used to refer to a whole “slice” of the medium, and the position of that slice, along the \(x\) axis, at the time \(t\) will be given by \(x+\xi(x, t)\). In both of these cases, the displacement vector \(\xi\) reduces to a single nonzero component (along the \(y\) or \(x\) axis, respectively), which can, of course, be positive or negative. I will restrict myself implicitly to these simple cases and treat \(\xi\) as a scalar from this point on.
Under these conditions, the function \(\xi(x, t)\) (which is often called the wave function ) gives us the shape of the “displacement wave,” that is to say, the displacement of every part of the medium, labeled by its equilibrium \(x\)-coordinate, at any instant in time. Accordingly, taking the derivative of \(\xi\) gives us the velocity of the corresponding part of the medium:
\[ v_{\operatorname{med}}=\frac{d \xi}{d t} \label{eq:12.2} .\]
This is also, in general, a vector (along the direction of motion of the wave, if the wave is longitudinal, or perpendicular to it if the wave is transverse). It is also a function of time, and in general will be different from the speed of the wave itself , which we have taken to be constant, and which I will denote by \(c\) instead.
Harmonic Waves
An important class of waves are those for which the wave function is sinusoidal. This means that the different parts of the medium execute simple harmonic motion, all with the same frequency, but each (in general) with a different phase. Specifically, for a sinusoidal wave we have
\[ \xi(x, t)=\xi_{0} \sin \left[\frac{2 \pi x}{\lambda}-2 \pi f t\right] \label{eq:12.3} .\]
In Equation (\ref{eq:12.3}), \(f\) stands for the frequency, and plays the same role it did in the previous chapter: it tells us how often (that is, how many times per second) the corresponding part of the medium oscillates around its equilibrium position. The constant \(\xi_0\) is just the amplitude of the oscillation (what we used to call \(A\) in the previous chapter). The constant \(\lambda\), on the other hand, is sometimes known as the “spatial period,” or, most often, the wavelength of the wave: it tells you how far you have to travel along the \(x\) axis, from a given point \(x\), to find another one that is performing the same oscillation with the same amplitude and phase.
A couple of snapshots of a harmonic wave are shown in Figure \(\PageIndex{3}\). The figure shows the displacement \(\xi\), at two different times, and as a function of the coordinate \(x\) used to label the parts into which we have broken up the medium (as explained in the previous subsection). As such, the wave it represents could equally well be longitudinal or transverse. If it is transverse, like a wave on a string, then you can think of \(\xi\) as being essentially just \(y\), and then the displacement curve (the blue line) just gives you the shape of the string. If the wave is longitudinal, however, then it is a bit harder to visualize what is going on just from the plot of \(\xi (x, t)\). This is what I have tried to do with the density plots at the bottom of the figure.
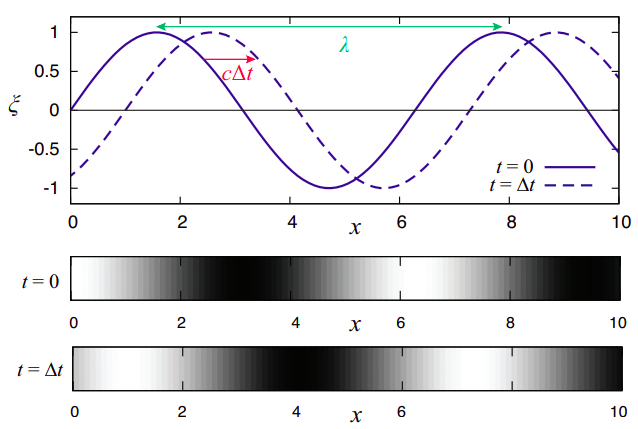
Imagine the wave is longitudinal, and consider the \(x = \pi\) point on the \(t\) = 0 curve (the first zero, not counting the origin). A particle of the medium immediately to the left of that point has a positive displacement, that is, it is pushed towards \(x = \pi\), whereas a slice on the right has a negative displacement—which means it is also pushed towards \(x = \pi\). We therefore expect the density of the medium to be highest around that point, whereas around \(x = 2\pi\) the opposite occurs: particles to the left are pushed to the left and those to the right are pushed to the right, resulting in a low-density region. The density plot labeled \(t\) = 0 attempts to show this using a grayscale where darker and lighter correspond to regions of higher and smaller density, respectively. At the later time \(t = \Delta t\) the high and low density regions have moved a distance \(c\Delta t\) to the right, as shown in the second density plot.
Regardless of whether the wave is longitudinal or transverse, if it is harmonic, the spatial pattern will repeat itself every wavelength; you can think of the wavelength \(\lambda\) as the distance between two consecutive crests (or two consecutive troughs) of the displacement function, as shown in the figure. If the wave is traveling with a speed \(c\), an observer sitting at a fixed point \(x\) would see the disturbance pass through that point, the particles move up and down (or back and forth), and the motion repeat itself after the wave has traveled a distance \(\lambda\), that is, after a time \(\lambda/c\). This means the period of the oscillation at every point is \(T = \lambda/c\), and the corresponding frequency \(f = 1/T = c/ \lambda\):
\[ f=\frac{c}{\lambda} \label{eq:12.4} .\]
This is the most basic equation for harmonic waves. Making use of it, Equation (\ref{eq:12.3}) can be rewritten as
\[ \xi(x, t)=\xi_{0} \sin \left[\frac{2 \pi}{\lambda}(x-c t)\right] \label{eq:12.5} .\]
This suggests that if we want to have a wave moving to the left instead, all we have to do is change the sign of the term proportional to \(c\), which is indeed the case.
In contrast to the wave speed, which is a constant, the speed of any part of the medium, with equilibrium position \(x\), at the time \(t\), can be calculated from Eqs. (\ref{eq:12.2}) and (\ref{eq:12.3}) to be
\[ v_{m e d}(x, t)=2 \pi f \xi_{0} \cos \left[\frac{2 \pi x}{\lambda}-2 \pi f t\right]=\omega \xi_{0} \cos \left[\frac{2 \pi x}{\lambda}-2 \pi f t\right] \label{eq:12.6} \]
(where I have introduced the angular frequency \(\omega = 2\pi f\)). Again, this is a familiar result from the theory of simple harmonic motion: the velocity is “90 degrees out of phase” with the displacement, so it is maximum or minimum where the displacement is zero (that is, when the particle is passing through its equilibrium position in one direction or the other).
Note that the result (\ref{eq:12.6}) implies that, for a longitudinal wave, the “velocity wave” is in phase with the “density wave” : that is, the medium velocity is large and positive where the density is largest, and large and negative where the density is smallest (compare the density plots in Figure \(\PageIndex{3}\)). If we think of the momentum of a volume element in the medium as being proportional to the product of the instantaneous density and velocity, we see that for this wave, which is traveling in the positive \(x\) direction, there is more “positive momentum” than “negative momentum” in the medium at any given time (of course, if the wave had been traveling in the opposite direction, the sign of \(v_{med}\) in Equation (\ref{eq:12.6}) would have been negative, and we would have found the opposite result). This confirms our expectation that the wave carries a net amount of momentum in the direction of propagation. A detailed calculation (which is beyond the scope of this book) shows that the time-average of the “momentum density” (momentum per unit volume) can be written as
\[ \frac{p}{V}=\frac{1}{2 c} \rho_{0} \omega^{2} \xi_{0}^{2} \label{eq:12.7} \]
where \(\rho_{0}\) is the medium’s average mass density (mass per unit volume). Interestingly, this result applies also to a transverse wave!
As mentioned in the introduction, the wave also carries energy. Equation (\ref{eq:12.6}) could be used to calculate the kinetic energy of a small region of the medium (with volume \(V\) and density \(\rho_{0}\), and therefore \(m=\rho_{0} V\)), and its time average. This turns out to be equal to the time average of the elastic potential energy of the same part of the medium (recall that we had the same result for harmonic oscillators in the previous chapter). In the end, the total time-averaged energy density (energy per unit volume) in the region of the medium occupied by the wave is given by
\[ \frac{E}{V}=\frac{1}{2} \rho_{0} \omega^{2} \xi_{0}^{2} \label{eq:12.8} .\]
Comparing (\ref{eq:12.7}) and (\ref{eq:12.8}), you can see that
\[ \frac{E}{V}=\frac{c p}{V} \label{eq:12.9} .\]
This relationship between the energy and momentum densities (one is just \(c\) times the other) is an extremely general result that applies to all sorts of waves, including electromagnetic waves!
The Wave Velocity
You may ask, what determines the speed of a wave in a material medium? The answer, qualitatively speaking, is that \(c\) always ends up being something of the form
\[ c \sim \sqrt{\frac{\text { stiffness }}{\text { inertia }}} \label{eq:12.10} \]
where “stiffness” is some measure of how rigid the material is (how hard it is to compress it or, in the case of a transverse wave, shear it), whereas “inertia” means some sort of mass density.
For a transverse wave on a string, for instance, we find
\[ c=\sqrt{\frac{F t}{\mu}} \label{eq:12.11} \]
where \(F^t\) is the tension in the string and \(\mu\) is not the “reduced mass” of anything (sorry about the confusion!), but a common way to write the “mass per unit length” of the string. We could also just write \(\mu = M/L\), where \(M\) is the total mass of the string and \(L\) its length. Note that the tension is a measure of the stiffness of the string, so this is, indeed, of the general form (\ref{eq:12.10}). For two strings under the same tension, but with different densities, the wave will travel more slowly on the denser one.
For a sound wave in a fluid (liquid or gas), the speed of sound is usually written
\[ c=\sqrt{\frac{B}{\rho_{0}}} \label{eq:12.12} \]
where \(\rho_0\) is the regular density (mass per unit volume), and \(B\) is the so-called bulk modulus , which gives the fluid’s resistance to a change in volume when a pressure \(P\) is applied to it: \(B = P/(\Delta V /V )\). So, once again, we get something of the form (\ref{eq:12.10}). In this case, however, we find that for many fluids the density and the stiffness are linked, so they increase together, which means we cannot simply assume that the speed of sound will be automatically smaller in a denser medium. For gases, this does work well: the speed of sound in a lighter gas, like helium, is greater than in air, whereas in a denser gas like sulfur hexafluoride the speed of sound is less than in air 1 . However, if you compare the speed of sound in water to the speed of sound in air, you find it is much greater in water, since water is much harder to compress than air: in this case, the increase in stiffness more than makes up for the increase in density.
The same thing happens if you go from a liquid like water to a solid, where the speed of sound is given by
\[ c=\sqrt{\frac{Y}{\rho_{0}}} \label{eq:12.13} \]
where \(Y\) is, again, a measure of the stiffness of the material, called the Young modulus . Since a solid is typically even harder to compress than a liquid, the speed of sound in solids such as metals is much greater than in water, despite their being also denser. For reference, the speed of sound in steel would be about \(c\) = 5,000 m/s; in water, about 1,500 m/s; and in air, “only” about 340 m/s.
1 This effect can be used to produce “funny voices,” because of the relationship \(f = c/\lambda\) (Equation (\ref{eq:12.4})), which will be discussed in greater detail in the section on standing waves.
Reflection and Transmission of Waves at a Medium Boundary
Suppose that you have two different elastic media, joined in some way at a common boundary, and you have a wave in the first medium traveling towards the boundary. Examples of media connected this way could be two different strings tied together, or two springs with different spring constants joined at the ends; or, for sound waves, it could just be something like water with air above it: a compression wave in air traveling towards the water surface will push on the surface and set up a sound wave there, and vice-versa.
The first thing to notice is that, if the incident wave has a frequency \(f\), it will cause the medium boundary, when it arrives there, to oscillate at that frequency. As a result of that, the wave that is set up in the second medium—which we call the transmitted wave —will also have the same frequency \(f\). Again, think of the two strings tied together, so the first string “drives” the second one at the frequency \(f\); or the sound at the air-water boundary, driving (pushing) the water surface at the frequency \(f\).
So, the incident and transmitted waves will have the same frequency, but it is clear that, if the wave speeds in the two media are different, they cannot have the same wavelength: since the relation (\ref{eq:12.4}) has to hold, we will have \(\lambda_1 = c_1/f\), and \(\lambda_2 = c_2/f\). Thus, if a periodic wave goes from a slower to a faster medium, its wavelength will increase, and if it goes from a faster to a slower one, the wavelength will decrease.
It is easy to see physically why this happens, and how it has to be the case even for non-periodic waves, that is, wave pulses: a pulse going into a faster medium will widen in length (stretch), whereas a pulse going into a slower medium will become narrower (squeezed). Imagine, for example, several people walking in line, separated by the same distance \(d\), all at the same pace, until they reach a line beyond which they are supposed to start running. When the first person reaches the line, he starts running, but the second one is still walking, so by the time the second one reaches the line the first one has increased his distance from the second. The same thing will happen between the second and the third, and so on: the original “bunch” will become spread out. (If you watch car races, chances are you have seen this kind of thing happen already!)
Besides setting up a transmitted wave, with the properties I have just discussed, the incident wave will almost always cause a reflected wave to start traveling in the first medium, moving backwards from the boundary. The reflected wave also has the same frequency as the incident one, and since it is traveling in the same medium, it will also have the same wavelength. A non-periodic pulse, when reflected, will therefore not be stretched or squeezed, but it will be “turned around” back-to-front, since the first part to reach the boundary also has to be the first to leave. See Figure \(\PageIndex{4}\) (the top part) for an example.
What is the physical reason for the reflected wave? Ultimately, it has to do with the energy carried by the incident wave, and whether it is possible for the transmitted wave alone to handle the incoming energy flux or not. As we saw earlier (Equation (\ref{eq:12.8})), the energy per unit volume in a harmonic wave of angular frequency \(\omega\) and amplitude \(\xi_0\) is \(E/V = \frac{1}{2} \rho_{0}\omega^{2}\xi^{2}_{0}\). If the wave is traveling at a speed \(c\), then the energy flux (energy transported per unit time per unit area) is equal to \((E/V )c\), which is to say
\[ I=\frac{1}{2} c \rho_{0} \omega^{2} \xi_{0}^{2} \label{eq:12.14} .\]
This is often called the intensity of the wave. It can be written as \(I=\frac{1}{2} Z \omega^{2} \xi_{0}^{2}\), where I have defined the medium’s mechanical impedance (or simply the impedance ) as
\[ Z = c \rho_{0} \label{eq:12.15} \]
(for a string, the mass per unit length \(\mu\) instead of the mass per unit volume \(\rho_0\) should be used). You can see that if the two media have the same impedance, then the energy flux in medium 2 will exactly match that in medium 1, provided the incident and transmitted waves have the same amplitudes. In that case, there will be no reflected wave: even if the two media have different densities and wave velocities, as long as they have the same impedance, the wave will be completely transmitted.
On the other hand, if the media have different impedances, then it will in general be impossible to match the energy flux with only a transmitted wave, and reflection will occur. This is not immediately obvious, since it looks like all you have to do, to compensate for the different impedances in Equation (\ref{eq:12.14}), is to give the transmitted wave an amplitude that is different from that of the incident wave. But the point is precisely that, mathematically, you cannot do that without introducing a reflected wave. This is because the actual amplitude of the oscillation at the boundary has to be the same on both sides, since the two media are connected there, and oscillating together; so, if \(\xi_{0, \text { inc }}\) is going to be different from \(\xi_{0, \text { trans }}\), you need to have another wave in medium 1, the reflected wave, to insure that \(\xi_{0, \text { inc }}+\xi_{0, \text { refl }}=\xi_{0, \text { trans }}\).
Another way to see this is to dig in a little deeper into the physical meaning of the impedance. This is a worthwhile detour, because impedance in various forms recurs in a number of physics and engineering problems. For a sound wave in a solid, for instance, we can see from Eqs. (\ref{eq:12.13}) and (\ref{eq:12.15}) that \(Z = c\rho_0 = \sqrt{Y \rho_0}\); so a medium can have a large impedance either by being very stiff (large \(Y\)) or very dense (large \(\rho_0\)) or both; either way, one would have to work harder to set up a wave in such a medium than in one with a smaller impedance. On the other hand, once the wave is set up, all that work gets stored as energy of the wave, so a wave in a medium with larger \(Z\) will also carry a larger amount of energy (as is also clear from Equation (\ref{eq:12.14})) 2 for a given displacement \(\xi_0\).
So, when a wave is trying to go from a low impedance to a large impedance medium, it will find it hard to set up a transmitted wave: the transmitted wave amplitude will be small (compared to that of the incident wave), and the only way to satisfy the condition \(\xi_{0, \text { inc }}+\xi_{0, \text { refl }}=\xi_{0, \text { trans }}\) will be to set up a reflected wave with a negative amplitude 3 —in effect, to flip the reflected wave upside down, in addition to left-to-right. This is the case illustrated in the bottom drawing in Figure \(\PageIndex{4}\).
Conversely, you might think that a wave trying to go from a high impedance to a low impedance medium would have no trouble setting up a transmitted wave there, and that is true—but because of its low impedance, the transmitted wave will still not be able to carry all the energy flux by itself. In this case, \(\xi_{0,trans}\) will be greater than \(\xi_{0,inc}\), and this will also call for a reflected wave in the first medium, only now it will be “upright,” that is, \(\xi_{0, \text { refl }}=\xi_{0, \text { trans }}-\xi_{0, \text { inc }}>0\).
To finish up the subject of impedance, note that the observation we just made, that impedance will typically go as the square root of the product of the medium’s “stiffness” times its density, is quite general. Hence, a medium’s density will typically be a good proxy for its impedance, at least as long as the “stiffness” factor is independent of the density (as for strings, where it is just equal to the tension) or, even better, increases with it (as is typically the case for sound waves in most materials). Thus, you will often hear that a reflected wave is inverted (flipped upside down) when it is reflected from a denser medium, without any reference to the impedance—it is just understood that “denser” also means “larger impedance” in this case. Also note, along these lines, that a “fixed end,” such as the end of a string that is tied down (or, for sound waves, the closed end of an organ pipe), is essentially equivalent to a medium with “infinite” impedance, in which case there is no transmitted wave at that end, and all the energy is reflected.
Finally, the expression \(\xi_{0,inc} + \xi_{0,refl}\) that I wrote earlier, for the amplitude of the wave in the first medium, implicitly assumes a very important property of waves, which is the phenomenon known as interference , or equivalently, the “linear superposition principle.” According to this principle, when two waves overlap in the same region of space, the total displacement is just equal to the algebraic sum of the displacements produced by each wave separately. Since the displacements are added with their signs, one may get destructive interference if the signs are different, or constructive interference if the signs are the same. This will play an important role in a moment, when we start the study of standing waves.
2 In this respect, it may help you to think of the impedance of an extended medium as being somewhat analog to the inertia (mass) of a single particle. The larger the mass, the harder it is to accelerate a particle, but once you have given it a speed v, the larger mass also carries more energy.
3 A better way to put this would be to say that the amplitude is positive as always, but the reflected wave is 180\(^{\circ}\) out of phase with the incident wave, so the amplitude of the total wave on the medium 1 side of the boundary is \(\xi_{0,inc} − \xi_{0,refl}\).
Advertisement
Do tornadoes always move from west to east?
- Share Content on Facebook
- Share Content on LinkedIn
- Share Content on Flipboard
- Share Content on Reddit
- Share Content via Email

Key Takeaways
- While most tornadoes move from southwest to northeast, there's no absolute rule.
- Tornadoes are influenced by the thunderstorms that form them, following the general path of these storms, but they can suddenly turn or reverse direction due to specific wind conditions.
A good rule of thumb when learning about tornadoes : There's no such thing as always. Sure, some rules aren't easily broken; lying low in an interior room bathroom tub, for instance, is going to be a lot safer than riding out a tornado in a mobile home. But don't fool yourself into thinking that the bathtub is the perfect safe haven, as 200-mile-an-hour (322-kilometer-an-hour) winds can fling them about pretty easily [source: Edwards ]. Likewise, cities aren't often hit by tornadoes, but best you not go spouting off to people in Oklahoma City that they're safe from a twister. Many cities have been hit hard by tornadoes -- including OKC, multiple times-- and there's no reason to believe they are "safer."
All this is to say that if you see a tornado, don't assume that running west is going to save you. While most tornadoes do move from southwest to northeast, that isn't some sort of law of nature [source: MCEG]. In fact, tornadoes must be routinely amused by what lowly humans assume are their "natural laws," because they also don't fear bodies of water or mountains, and nothing "attracts" them to trailer parks.
Just because most tornadoes move in this west-to-east formation doesn't mean that all of them do. Remember that they're formed by thunderstorms , so they often follow the path of their "parent" storms, which also generally move from the southwest [sources: Cappella ; Chicago Tribune ]. But tornadoes can easily turn or even backpedal -- sometimes quite suddenly -- and travel the opposite way if they're hit with the right kind of wind from a system [source: City of Chicago ].
Do remember that different places in the United States (and the world in general) have their own specific, picky weather patterns. Minnesota, for instance, will often have storms that move in a northwestern direction, which checks out, because a lot of their weather systems come down from the north. Texas gets a lot of southeastern fronts, so they're going to see different paths on their storms [source: MCEG]. Each region of the world might have a general weather or storm pattern, but -- maybe you've heard -- weather is unpredictable. It's really dangerous to assume that, just because the storms or wind in your area tend to move in one direction, you can determine where a storm is going.
Bottom line? Tornadoes will often move in a generally west-to-east pattern through a lot of tornado country. But that doesn't mean that they always do, nor does it guarantee that they'll steadfastly stay the course. If you're running from a tornado, best you run straight to a shelter and not try to predict where it will mosey off.
Frequently Asked Questions
What factors influence the direction of a tornado, can tornadoes cross over bodies of water, lots more information, related articles.
- How Fire Tornadoes Work
- 10 Myths About Surviving a Tornado
- How Tornadoes Work
- Can NASA predict natural disasters?
- Name the Price: Natural Disasters
- Shocking: The Thunderstorm Danger Quiz
- Cappella, Chris. "Answers Archive: Tornado Science." USA Today. Sept. 21, 2003. (Dec. 31, 2014) http://usatoday30.usatoday.com/weather/resources/askjack/archives-tornado-science.htm
- Chicago Tribune. "Ask Tom Why." July 30, 2011. (Dec. 31, 2014) http://articles.chicagotribune.com/2011-07-30/news/ct-wea-0731-asktom-20110730_1_tornadoes-thunderstorms-move-parent-thunderstorms
- City of Chicago. "The Basics About Tornadoes." (Dec. 31, 2014) https://webapps.cityofchicago.org/ChicagoAlertWeb/contents/ext/tornado/tornado_basics.jsp
- Edwards, Roger. "The Online Tornado FAQ." Storm Prediction Center at the National Oceanic and Atmospheric Administration. March 5, 2015. (Dec. 31, 2014) http://www.spc.noaa.gov/faq/tornado/
- Milwaukee County Emergency Government, et al. (MCEG). "Tornado Safety." University of Wisconsin Milwaukee. (Dec. 31, 2014) https://www4.uwm.edu/usa/safety/emergency_preparedness/funnel_facts.cfm
Please copy/paste the following text to properly cite this HowStuffWorks.com article:

COMMENTS
September 30, 2016 at 6:15 am. Throughout the universe, it's natural for energy to flow from one place to another. And unless people interfere, thermal energy — or heat — naturally flows in one direction only: from hot toward cold. Heat moves naturally by any of three means. The processes are known as conduction, convection and radiation.
The most likely explanation for why time only moves in one direction is the Second Law of Thermodynamics, which states that in an isolated system, entropy can only increase. "It's a mercy that time runs in one direction only, that we see the past but darkly and the future not at all.". - Olivia Laing.
A thermochemical calorie is defined as the amount of energy required to heat up 1.00 gram of liquid water from 14.5°C to 15.5°C, at 1.00 atmosphere of ambient pressure — i.e., at sea level on a fair day. One thermochemical calorie, 1.00 cal, is equivalent to 4.184 Joules.
Entropy is one of the few quantities in the physical sciences that require a particular direction for time, sometimes called an arrow of time. As one goes "forward" in time, the second law of thermodynamics says, the entropy of an isolated system can increase, but not decrease. Thus, entropy measurement is a way of distinguishing the past from ...
Heat flows from hot to cold because warmer molecules move faster. When warmer molecules bump into cooler ones, two things change: cooler molecules speed up (get warmer), and warmer molecules slow down (get cooler.) The flow of kinetic energy from warmer objects to cooler objects is "conduction.". Keep reading to find out more about this ...
Heat energy always flows from the warmer object to the cooler object. Heat always flows from a warmer object to a cooler object (Let's Talk Science using an image by VectorMine via iStockphoto ). Image - Text Version. Heat can be transferred in three ways: Conduction. Convection. Radiation. Boiling water in a kettle on the stove is a good ...
The kinetic theory helps us understand where the energy goes when we heat something up. If you put a pan full of cold water on a hot stove, you're going to make the molecules in the water move around more quickly. The more heat you supply, the faster the molecules move and the further apart they get.
Here's what to do: Add hot water to one cup and cold water to another cup until they are each about half-full. In a separate empty cup, make a detergent solution by mixing ½ teaspoon of liquid dish detergent with 1 tablespoon of water. Lower the open mouth of the bottle into the cup with detergent. Carefully tilt and lift the bottle out so ...
The Earth's two great continental glaciers, on Antarctica and Greenland, comprise about 99% of all of the world's glacial ice, and approximately 68% of all of the Earth's fresh water. As is evident from Figure 4.2.2, the Antarctic ice sheet is vastly bigger than the Greenland ice sheet; it contains about 17 times as much ice.
A glacier is a huge mass of ice that moves slowly over land. The term "glacier" comes from the French word glace (glah-SAY), which means ice.Glaciers are often called " rivers of ice." Glaciers fall into two groups: alpine glaciers and ice sheets. Alpine glaciers form on mountainsides and move downward through valleys.Sometimes, alpine glaciers create or deepen valleys by pushing dirt ...
As a result, it is unusual for icebergs to move in the same direction as sea ice. Typically, their direction of motion relative to the surface wind is some 40°-50° to the right (Northern Hemisphere) or left (Southern Hemisphere). Icebergs progress at about 3 percent of the wind speed. Iceberg scour and sediment transport
On large ice shelves, it can take more than a thousand years for an ice crystal to travel through the entire ice shelf and commence the final stage of its journey aboard an iceberg. Ice shelves in the Antarctic are, as a rule, between 300 and 2500 metres thick, although they become thinner the further they extend out into the sea.
My solution has been, (wifey hates riding "backwards"), is to book seats across a table or in a compartment. One of us will always be riding in the right direction (her), and we can switch if the train does reverse along the way. Also, ICE trains have electronic signs over the seats indicating if the seat has been reserved and between what ...
19. Air does indeed flow from high pressure to low pressure area (see the wind arrows on a weather chart), but in the case of two rooms the much more important effect is that of warm thinner air rising towards the ceiling when the air from the two rooms gets mixed. Thus, cold air from the cold room will be leaving the room close to the floor ...
Electromagnetic radiation is one of the many ways that energy travels through space. The heat from a burning fire, the light from the sun, the X-rays used by your doctor, as well as the energy used to cook food in a microwave are all forms of electromagnetic radiation. While these forms of energy might seem quite different from one another ...
Now, cut the ice to the desired size. Using a sturdy serrated knife, score grooves into the ice, about 1 centimeter deep, and then tap the back of the blade with a hammer to break through the rest ...
On a completely clear day over smooth sea ice, most sunlight would reflect back into the atmosphere, which is one way that sea ice cools the planet. But when the ice has ridges or darker melt ponds — or is dotted with pollutants — it can change the equation, increasing the amount of ice melt.
Light - Reflection, Refraction, Physics: Light rays change direction when they reflect off a surface, move from one transparent medium into another, or travel through a medium whose composition is continuously changing. The law of reflection states that, on reflection from a smooth surface, the angle of the reflected ray is equal to the angle of the incident ray. (By convention, all angles in ...
In truth, while t northeast is the most common direction for U.S. tornadoes, they can travel in any direction. In one famous case in 1997 an extremely powerful and deadly F5 tornado traveled ...
As we saw earlier (Equation ( 12.1.8 )), the energy per unit volume in a harmonic wave of angular frequency ω and amplitude ξ0 is E / V = 1 2ρ0ω2ξ2 0. If the wave is traveling at a speed c, then the energy flux (energy transported per unit time per unit area) is equal to (E / V)c, which is to say. I = 1 2cρ0ω2ξ2 0.
However, the reason behind clouds moving in the opposite direction may be the surface friction slowing the wind. This may eventually cause a shift in the direction of the current and near the surface. So, the cloud movement may differ from the wind direction during that point. You may also notice that the clouds close to the ground are moving ...
Key Takeaways. While most tornadoes move from southwest to northeast, there's no absolute rule. Tornadoes are influenced by the thunderstorms that form them, following the general path of these storms, but they can suddenly turn or reverse direction due to specific wind conditions. A good rule of thumb when learning about tornadoes: There's no ...