Site Monitor Visits
Study sites are monitored to ensure oversight of the clinical research study by the sponsor..
Regular site monitor visits can be broken down into four types: pre-study visits, initiation visits, periodic monitoring visits, and close-out visits. Study sites may also be monitored or audited by the FDA, Clinical Research Organizations (CROs), IRBs and sponsors. For more information on site audits by outside entities please visit the training documents on Quality Management: Audits .

Pre-Study Visits
Pre-study visits (site selection visits or site qualification visits (SQVs)) are conducted to determine if the investigator and clinical site have the capability to conduct the study. During this visit, both an investigator and a study coordinator must be available. When applicable, pharmacy staff may also need to be available. The monitor will usually request a tour of the facility and time to discuss the basic fundamentals of the protocol and how that relates to the feasibility of recruiting potential participants.
Other topics of discussion during the pre-study visit include:
- Investigator responsibilities
- Qualifications of investigator or other site personnel
- Study objectives, protocol-required procedures, eligibility criteria, and patient recruitment
- IRB (e.g., informed consent requirements)
- Adverse event reporting, source documentation, and record retention
- Space requirements, availability of a secure area to store investigations drug or devices, availability of required equipment
Initiation Visits
A Site Initiation Visit (SIV) is when the research study team receives adequate training from the sponsor or CRO on the protocol. It is also the opportunity for the sponsor or CRO to ensure that the investigator fully understands his/her responsibilities (21 CFR 312 Subpart D). This visit usually occurs after the site has completed all regulatory requirements and has obtained IRB approval for the research study at their site. The initiation visit is the last step before the study site is activated for enrollment by the sponsor.
During study start-up, a sponsor may choose to hold an investigator meeting for a large number of sites in lieu of conducting many site initiation visits. If you are required to travel to an investigator meeting for a study, visit the Office of Sponsored Programs (OSP) for OSU’s travel policies.
Other topics of discussion during the site initiation visit include:
- Study overview, eligibility criteria, procedures, access to suitable patient population
- Lab manual, requirements for research sample processing and shipping
- Regulations and Good Clinical Practice (GCP) guidelines, informed consent requirements, IRB obligations, adverse event reporting, drug accountability
- Data forms review including Case Report Forms (CRFs)
- Regulatory documents and study file organization
- 21 CFR 312 Subpart D Responsibilities of Sponsors and Investigators
- OSP Travel
Periodic Monitoring Visits
The sponsor and/or CRO will develop a monitor plan that includes the frequency and duration of periodic monitor visits. The focus of these visits is to evaluate the way the study is being conducted and to perform source document verification. These visits can occur every few weeks to once a year and can take less than one day up to several days at a time. It is important to be familiar with the contract agreements, as they may contain the agreement as to the frequency and duration of site monitoring visits and the sponsor’s data entry expectations. The contract agreement should match what the sponsor and/or CRO creates in the site monitor plan.
At the beginning of the study, it should be determined where the monitor or monitors will work while they are on site. Ideally, the monitor should have their own workspace separate from the research study team. In addition, many monitors require internet access for their laptops. Instructions for OSUMC guest wireless can be found here .
Preparing for a periodic monitoring visit:
- Identify a quiet place for the monitor to work and ensure access to a copy machine, phone, water fountain, and restroom
- Complete all necessary CRFs
- Confirm that Serious Adverse Event (SAE) forms have been submitted and are available for review
- Obtain medical records for the CRFs to be reviewed
- Organize study file documents for review
- Confirm that signed consent forms for all enrolled participants are available
- Schedule an appointment for the monitor to visit the pharmacy if needed
- Schedule time for the study coordinator to meet with the monitor towards the end of the visit to review findings
- Schedule time for the investigator to meet with the monitor towards the end of the visit to review findings
Close-Out Visits
When the research study has been completed at a site, a close-out visit occurs. This type of visit can take the form of an on-site visit or, in some cases, be conducted via a telephone call. Some close-out visits are also combined with a final periodic monitoring visit.
Action items during the close-out visit may include:
- Discuss timelines and strategies for the completion of outstanding case report forms and queries
- Return or destruction of study drug
- Collect outstanding patient data forms and study forms such as the screening and monitoring logs
- Perform a final review of the study file documents
- Discuss the plans for record retention
- Discuss ongoing investigator responsibilities
Liu, M.B. and Davis, K.; Chapter 6: Monitoring. Lessons from a Horse Named Jim: a Clinical Trials Manual from the Duke Clinical Research Institute . Durham, NC: Duke Clinical Research Institute, 2001. Print.
If you have a disability and experience difficulty accessing this content, please submit an email to [email protected] for assistance.
- Technical Help
- CE/CME Help
- Billing Help
- Sales Inquiries
Best Practices for Clinical Site Selection
Practical and Protocol Considerations
Recruitment and retention strategies, regulatory considerations, references:.
Clinical trials are expensive and labor-intensive. Failures within the clinical trial process result in a huge loss of valuable resources and time. [ 1 ] These challenges can stall a clinical trial and delay life-saving medical therapies and devices from entering the healthcare marketplace. Statistics indicate that an overwhelming 80% of clinical studies are not able to meet their enrollment timelines and objectives because of recruitment difficulties. [ 1 , 6 ]
By implementing a series of best practices for clinical site selection, study sponsors can mitigate potential problems. This is accomplished by carefully navigating several key factors involving population profiles, safety, recruitment strategies, funding [ 5 ], and regulatory controls. [ 1 ] Notably, industry experts cite a strong correlation between meeting recruitment goals and clinical site selection. [ 6 ]
Globally, more than 80% of trials fail to enroll on time resulting in an extension of study and or addition of new study sites. [ 13 ]
Subsequently, principal investigators and sponsors place heavy emphasis on identifying site locations with ample resources, such as staff expertise and access to the target subject population. [ 1 , 2 , 3 ] Principal investigators work to optimize clinical trial volunteer engagement and improve protocol adherence, thereby ensuring a successful trial experience for study subjects. Successful clinical sites benefit from past experiences, as survey-based studies indicate that repeated use of clinical sites is common. [ 3 , 6 ] This is especially true if the clinical trial site can demonstrate a track record of meeting enrollment targets and successfully completing studies. [ 2 , 3 ]
Implementing Best Practices in Clinical Site Selection
Research sites are ramping up their efforts to combat the many challenges associated with site selection by using prior knowledge, proven practices, and enhanced technology. Taking strategic steps to determine early site feasibility [ 8 ] is recommended and should include the following:
- Is the study site situated in an accessible location for study participants?
- Can study visits be decentralized and brought closer to participants?
- Are there measures in place to lower premature dropout rates due to distance?
- What strategies are in place to identify patient eligibility?
- Is there a correlation between the subject population and the disease or condition under study?
- Is the study protocol too complex?
- Is there a clinical site monitoring plan in place?
- Are vulnerable populations involved, such as the elderly, pediatric, or disabled? If so, will they have access to post-trial medical care?
- Are there economic or cultural aspects related to the potential clinical trial site that may deter eligible subjects from participating?
- Are fair selection practices being upheld?
- How are inclusion principles and disparity concerns being addressed?
- What are the enrollment objectives and allotted timeline?
- What is the maximum time allotted for study completion?
- Are there any competing or similar studies being conducted either at the same site or at another location?
- What historical takeaways from previously used clinical sites/teams need to be considered?
- Are there avenues for partnering with medical or social community members to help with recruitment efforts?
What site resources are important in a clinical trial site?
Sponsors must assess potential clinical trial sites for suitable facilities and resources. This is normally accomplished by conducting pre-site visits (PSVs) or site selection visits (SSVs). In preparing for a clinical trial, the details matter, such as maintaining the overall appearance of the potential site to ensure it is professional and welcoming to potential participants. Additionally, an ideal site will have adequate storage space, all needed clinical equipment, privacy options, and an attached or nearby research pharmacy. Flexible site access is also an integral component; participants favor facilities with weekend and late-evening appointments. [ 1 , 3 ]
Can staffing placements make a difference in clinical trial site selection?
Staffing appointments (existing and new) can significantly impact clinical trial site selection. Essential to the process, clinical site staff should be well-versed in Good Clinical Practice guidelines, federal and state regulations, and institutional policies. They should also demonstrate sincerity and compassion with trial participants. Because site coordinators and research nurses are tasked with vital duties such as data collection, they are often in constant contact with participants.
Patients’ perceptions regarding staff availability or interest (as well as having to meet with unfamiliar staff) can greatly impact recruitment and retention. [ 2 ] Patient satisfaction surveys suggest that the interactions between participants and clinical staff members play an influential role in clinical trial recruitment and overall experience . [ 7 ] Establishing patient trust by using consistent methods of communication and follow-thru often results in better participation and adherence.
Is there a risk to using clinical sites with internal site management teams already in place?
Studies concluded that clinical sites with a history of demonstrated site management operations offer the best results in terms of data collection and regulatory compliance. Regulatory duties may be outsourced to companies specializing in clinical site management. Although there is a tremendous benefit to streamlining the clinical process, the challenge of staff inconsistencies and turnover is ever-present. Common pitfalls include high investigator turnover (approximately 40% each year) because of a stressful site environment and burdens related to maintaining regulatory compliance. [10]
Research illustrates how clinical site selection can affect the success of a clinical study or trial. With many overlapping elements, staffing considerations affect multiple areas, including recruitment, retention, regulatory compliance, and overall patient experience. Utilizing best practices alongside a strategic pre-clinical site management plan is key when trying to meet recruitment objectives and study timelines.
As more technological enhancements come into play, we can expect more industry outsourcing to help manage data collection and reporting, in addition to incorporating biometrical data to help improve trial outcomes. While there are many benefits associated with outsourced clinical site management, research sponsors should approach each new potential clinical trial site selection with a fresh lens.
Industry thought leaders emphasize that there is no one-size fits all approach to site selection. By choosing clinical sites carefully and using proven practices, a study sponsor can increase the opportunities for a successful clinical trial.
- Hurtado-Chong, A., A. Joeris, D. Hess, and M. Blauth. 2017. “Improving site selection in clinical studies: a standardized, objective, multistep method and first experience results.” BMJ Open . 12;7(7): e014796. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5734283/ .
- Fogel, D.B. 2018. “Factors associated with clinical trials that fail and opportunities for improving the likelihood of success: A review.” 2018. Contemp Clin Trials Commun . 11:156-164. doi: 10.1016/j.conctc.2018.08.001.; https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6092479/ .
- Dombernowsky, T., M. Haedersdal, U. Lassen, et al . 2019. “Criteria for site selection in industry-sponsored clinical trials: a survey among decision-makers in biopharmaceutical companies and clinical research organizations.” Trials 20 ;708. https://doi.org/10.1186/s13063-019-3790-9
- Feyman Y., F. Provenzano, and F.S. David. 2020. “Disparities in Clinical Trial Access Across US Urban Areas.” JAMA Netw Open . 3(2): e200172. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7049079/ .
- DiMasi, J.A., H.G. Grabowski, and R.W. Hansen. 2015. “The cost of drug development.” N. Engl. J. Med. 372:1972. https://www.nejm.org/doi/10.1056/NEJMc1504317 .
- Bose, S.K., A. Sandhu, and S. Strommenger. 2017. “Clinical trials: A data driven feasibility approach.” Pharmaceutical Outsourcing. Blog. Feb. 1, http://www.pharmoutsourcing.com/Featured-Articles/333830-Clinical-Trials-A-Data-Driven-Feasibility-Approach/ .
- Thoma, A., F. Farrokhyar, L. McKnight, and M. Bhandar. 2010. “How to optimize patient recruitment.” Can. J. Surg. 53:205–210. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2878987/ .
- Rajadhyaksha V. 2010. “Conducting feasibilities in clinical trials: an investment to ensure a good study.” Perspect Clin Res . 1(3):106-9. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3146075/ .
- McMurray, J.J. 2016. “Site Selection and Performance in Clinical Trials.” Circulation: Heart Failure . 9: e003490. https://www.ahajournals.org/doi/full/10.1161/CIRCHEARTFAILURE.116.003490 .
- Getz, K.A., and M.J. Lamberti. 2013. “Global site landscape remains highly fragmented with variable performance.” Tufts Center for the Study of Drug Development Impact Report . 2013; 15 (1-3). March/April.
- Miller J. and J. Millum. 2022. “Ethical considerations in international clinical trial site selection.” Global Health 7: e008012. https://gh.bmj.com/content/bmjgh/7/4/e008012.full.pdf .
- Giutis, K., R.G. Hammermesh, and M. Krasnow. 2021. “Addressing demographic disparities in clinical trials.” Harvard Business Review . https://hbr.org/2021/06/addressing-demographic-disparities-in-clinical-trials .
- Desai M. 2020. “Recruitment and retention of participants in clinical studies: Critical issues and challenges.” Perspect Clin Res . 11(2):51-53. doi: 10.4103/picr.PICR_6_20. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7342339 /.

- On Campus Podcast – Title IX 2024 Regulation Updates
- What CITI Program is Reading – June 19, 2024
- Preview of the First State of the Science Address
- Pride Month Playlist: LGBTQIA+ Visibility in Research and Higher Ed
- Research Support Staff -Compliance
- RSP Compliance Coordinator
- Compliance Program Manager – Clinical Research
- Recruiting Manager
Privacy Overview

- Establish Clinical Sites
Help Industry With Clinical Trial Design
- Participate In Initial Industry-FDA Meetings
- Perform Patient Preference Studies
- Give Input On The Informed Consent Process
Site Selection
Your participation in choosing the site(s) for the clinical study can help mitigate patient risk and attract participants, as well as providing useful knowledge of day-to-day living and general practicalities to researchers. Geographic and travel information is vital to the site selection process. Researchers may also be unaware of certain travel barriers (motion sickness, frequent bathroom breaks) or issues of accessibility (no accessible transit available) that will affect your patient participation.
- An established Center of Excellence Network can simplify the site selection process.
- Areas where the target population lives and normally travels.
- Barriers travel may present.
- Special accommodations that may be needed at participating sites.
- It may be difficult for the patient’s partner, parents, or other family caregivers to maintain their jobs.
- If the family has children who are not participating in the study, the children’s daily care or school attendance may be affected.
- The participant or their family may be far from their support network.
- Financial burden may increase if the participants medical insurance provider may not be willing to cover medical costs that occur outside the study protocol because the site is out of network.
Continue to Step 3 >

- Joanna Thomson
- Sep 9, 2020
Site selection for clinical trials: a 2020 guide

Estimates suggest that recruitment difficulties make up about 45% of study delays every year . While these issues are down to a variety of factors, including a stigmatization of - and general disinterest in - clinical research, CROs and clinical trial sponsors look for research sites who can prove that they can successfully enroll, treat and evaluate participants. Careful consideration is essential for several ethical and operational reasons, and so research sites must take care in comprising clinical protocol that fits the needs of their investors.
Sites considered ‘inadequate’ by clinical trial sponsors could ultimately drain valuable resources through the extra training and the costs incurred by delays. In order to prevent these effects, trial organizers must ensure that potential sites are carefully screened and only those which are equipped and able to recruit patients and collect accurate, high-quality data are eventually selected. This will increase the likelihood of a trial that is both timely and cost-effective.
Today, we are giving investors and clinical research recruiters a brief guide on what to look for in research sites; and what research sites should be doing to make sure they impress. Check out our 2020 guide below.
What investors are looking for?
Most clinical research recruiters are well aware of the dangers of choosing wrongly, but simply do not know what they should look for when evaluating potential research sites. Here are some of the essential criteria to consider:
Staff experience:
To ensure clinical protocol compliance, it’s important to be sure that the right staff are available at each site. They should be experienced enough to be familiar with necessary compliance procedures, study conduct and clinical protocol. They should also be familiar with trial procedures such as the informed consent process, submissions to ethical and regulatory authorities and contracting.
Site history:
The screening of potential sites should always begin with a review of the site’s history. The only way to truly be sure the site can meet the requirements of the trial is to ensure it has previously been involved in a study of a similar size or complexity. Recruitment statistics for previous trials, if available, are also a great indicator of expected performance and should be compared to the estimates the site has provided.
Site capabilities:
Any thorough pre-screening of potential research sites should ensure that the site is equipped to fulfill all the activities specified in the clinical protocol. It is also important to determine whether they currently possess all the necessary infrastructure themselves or if some equipment needs to be provided. In this case, extra costs may be incurred, and delays will be caused.
Pre-registered patients:
If a site already has registered patients who meet the study criteria, this could be a significant benefit when it comes to meeting enrollment targets. However, it’s also important to ensure the site is not already involved in other similar studies which may compete for its patients and resources.
Site knowledge:
If a site specializes in a specific, target disease, they are more likely to be successful in recruiting patients and following protocol.
Travel is often a significant burden on patient recruitment and retention, and a barrier to patient participation. It is important to select research sites that are as close as possible to the target population, in order to achieve desired targets for enrollment and retention. This is especially crucial for those studies that require frequent site visits.
Something to think about
It is important for the design of the study and the protocol synopsis to be clear and finalized before the final selection is made. This ensures that site selection can be tailored to the demands of the study, and an appropriate selection can be made.
Utilising a CRO's network, experience and expertise can undoubtedly help in finding suitable sites and also improve the efficiency of the whole selection process.
Patient recruitment dashboards:
For research sites on a strict budget, the last thing sponsors want to do is waste time and money trying out various modes of recruitment and retention that just don’t stick. It’s a safe enough bet, for phase 1 clinical trials, to rely on traditional methods of recruitment (such as local clinic databases); but the fact is that these methods just don’t stand up in later stages of research. Today, clinical research recruiters should be looking to build relationships with a variety of patients from a wide-scale of geographical locations to ensure compliance in later trials; and the best way to do this is via established patient recruitment portals like Citrus .

Here at Citruslabs, we've created Citrus , the ideal fully-integrated patient recruitment and retention platform, to help any researcher: improve their current enrollment metrics with less effort , drive deeper communication , and boost effective retention that works .
Clinical trial enrollment i s notoriously difficult; this is why, every year, 15-20% of research sites fail to enroll a single patient! As most sites now realise, the key to improving these fragile statistics is by implementing a patient-centric approach to clinical research . A patient recruitment dashboard is your all-in-one solution to tackling this industry-wide issue by keeping the focus of your clinical studies on the patient.
Further, we ensure research sites are connected to a thoroughly educated and engaged pool of participants with over 3 million patients on record ; so, it is no wonder why we have such high patient confidence! Now, we would say that other models are available - but this would be a lie. In fact, unlike that offered by other patient recruitment companies , our easy-to-use platform is the first-of-its-kind for the market ; giving researchers a unique insight into their patients’ wants and needs via industry-leading technology. The future of clinical recruitment and retention starts here .
Interested in finding out more? Get in touch with us here , and check out our archives for all our top tips and tricks on running successful clinical trials in today's constantly changing industry.
Still a little unsure? Check out what our customers have to say about us here .
Recent Posts
Emerging Trends in Pet Health Research: What the Future Holds
Safe Beauty: Inside the World of Cosmetic Testing Labs
How to Boost Your Brand with Effective Pet Product Marketing Techniques

Introduction to the Monitoring in Clinical Trials, Pre-Study Site Selection Visit
- Post published: 31.07.2023

As per Good Clinical Practice (GCP) guideline “the purposes of trial monitoring are to verify that:
- a) The rights and well-being of human subjects are protected.
- b) The reported trial data are accurate, complete, and verifiable from source documents.
- c) The conduct of the trial is in compliance with the currently approved protocol/amendment(s), with GCP, and with the applicable regulatory requirement(s).”.
Clinical Trial monitoring is achieved via conducting the monitoring visits to Clinical Trial Sites (hereinafter referred to as “Sites”) either face-to-face or remotely.
The visits are performed by monitors (Clinical Research Associates, CRAs).
According to GCP guideline:
- “a) Monitors should be appointed by the sponsor.
- b) Monitors should be appropriately trained and should have the scientific and/or clinical knowledge needed to monitor the trial adequately. A monitor’s qualifications should be documented.
- c) Monitors should be thoroughly familiar with the investigational product(s), the protocol, written informed consent form and any other written information to be provided to subjects, the sponsor’s SOPs, GCP, and the applicable regulatory requirement(s).”.
The Sponsor of each Clinical Trial is responsible to develop a systematic, prioritized, risk-based approach to monitoring Clinical Trials. The Sponsor may choose on-site monitoring, a combination of on-site and centralized monitoring, or, where justified, centralized monitoring. The Sponsor should document the rationale for the chosen monitoring strategy (e.g., in the monitoring plan).
Centralized monitoring is a remote evaluation of accumulating data, performed in a timely manner, supported by appropriately qualified and trained persons (e.g., data managers, biostatisticians). Centralized monitoring processes provide additional monitoring capabilities that can complement and reduce the extent and/or frequency of on-site monitoring and help distinguish between reliable data and potentially unreliable data.
This article aims to briefly introduce the monitoring, describe the types of monitoring visits of Sites in Clinical Trials, and focus on a Pre-Study Site Selection Visit as a starting point in the monitoring process.
Depending on the timeframe (phase) in which each particular Clinical Trial Project is the following visits types are identified:
- Pre-Study Site Selection Visit (also known as Pre-Study Visit, Site Selection Visit, Site Qualification Visit)
- Site Initiation Visit
- Interim Monitoring Visit (also known as Routine Monitoring Visit)
- Close-out Visit.
Additionally, Co-Monitoring Visit(s) may be performed with the aim of either supporting the CRAs in their visit activities or assessing Site’s/CRAs’ adherence to Clinical Trial Protocol, GCP and other clinical research regulations. Co-Monitoring Visits are out of scope of this article.
Monitoring visits have typically one business day in duration, but depending on Clinical Trial complexity, amount of data to be reviewed and other factors their duration can be increased.
The main written documentation of each monitoring visit (irrespectively of its type) includes:
- Confirmation letter – a letter/e-mail sent to Site well in advance of planned monitoring visit and which includes the details and activities to be done during the visit
- Visit report – detailed description of all activities performed during the visit as well as all identified deficiencies, deviations, and action item(s)
- Follow-up letter – a letter/e-mail sent to Site after the visit has been conducted and which includes the details of all activities performed during the visit as well as all identified deficiencies, deviations, and action item(s).
All confirmation, follow-up letters and Site Initiation Visit Report (per GCP requirements) are to be properly stored in Investigator Site File located at Sites.
Pre-Study Site Selection Visit (PSSV)
This is the starting point of interactions between Sponsor/CRO and Clinical Trial Site. It is performed after the Site Identification & Feasibility process has been completed.
The PSSV is performed to ensure that:
- A potential Principal Investigator (PI) is qualified and interested in conducting the clinical study
- The PI’s Site has adequate facilities, and resources to properly complete all required study activities
- Site has required pool of patients to complete enrollment.
Items to be reviewed/discussed may include, but are not limited to:
- Feasibility Questionnaire completed by Site
- Clinical Study Protocol/Synopsis and Clinical Study timelines
- Enrollment (recruitment) target, strategy, expectations, and availability of subject population
- Qualifications/ training, experience, interest and availability of the PI and Site Staff performing study related duties
- PI and Site Staff obligations, ICH-GCP / ISO 14155 guidelines (if applicable) and regulatory requirements
- Therapeutic area being investigated (including applicable standard of care)
- The PI’s/Site Staff’s regulatory inspection/audit experience, if any, and the outcome of the inspection/audit(s)
- IRB/IEC requirements, documentation, and approval timelines
- Site budget and execution of Clinical Trial Agreements
- Informed Consent Process and documentation requirements
- Source document and study record requirements, as well as Source Data definitions and Good Documentation Practice
- Monitoring visit process/schedule, PI and Site Staff availability, other competitive clinical trials with the same pathology run by Site
- The monitoring strategy including remote monitoring and remote Source Data Verification capacity and acceptance (if applicable)
- Electronic Data Capture (EDC) requirements, including vendor-specific experience, internet connectivity, and computer availability, if applicable
- Turn-around time for data entry and data query resolution
- Local country / Site-specific requirements for clinical study conduct (e.g., Site SOPs)
- Reporting and documenting safety events (e.g., Adverse Events, Serious Adverse Events, Adverse Events of Special Interest, SUSARs, pregnancy, study drug overdose etc.)
- Medical management of study subjects remotely, if appropriate
- Assessment of storage area and conditions for Investigational Medical Product (IMP) (includes Investigational Medical Device (IMD) and/or other study supplies/materials)
- IMP/IMD Accountability procedures (receipt, storage, dispensing, and record keeping)
- Laboratory sample handling procedures, including supplies, collection, and shipment.
During PSSV CRA ensures the adequacy of facilities where study subjects will be seen by visiting (touring) them (e.g., exam rooms for subject evaluation and treatment, laboratory and any special testing area, pharmacy (if applicable), any satellite sites (if applicable), working area for Site staff, data entry area etc.) CRA also ensures the adequacy of available equipment to be used in clinical study (including validity check for calibration/maintenance documentation).
As part of PSSV CRA requests/collects any required Site documentation which may include, but is not limited to:
- Confidentiality (Non-Disclosure) Agreement, if applicable
- Medical licenses (Institution and Site staff) and dated/signed Curriculum Vitae (CVs)
- Current GCP and ISO 14155 (if applicable) training certificates or certificates of relevant training(s) as listed on the corresponding CVs
- IRB/IEC membership list (roster), SOPs (if applicable) and statement of compliance
- Electronic Health Records related documentation, if used
- Completed dated/signed Project-specific forms and questionnaires required to qualify the Site, as applicable
- Local laboratory reference ranges, accreditation certificates and CV of Head of local laboratory if it will be used
- Dated/signed attendees log, Clinical Study Protocol, and Investigator Brochure acceptance pages.
The above documents may be collected as originals or copies, depending on Sponsor’s/country specific requirements.
On the basis of conducted PSSV and the review of all applicable documentation the Sponsor either approves Site’s participation in the given Clinical Trial or declines it.
If Site is approved CRA sends an appropriate Site Selection Letter and in case of disapproval – the appropriate Site Non-Selection Letter.
The above letters will thank PI and Site Staff for completion of Selection activities and will state the further actions to be completed (if Site is approved) or will explain the reason(s) for non-selection decision.
Carpathian Research Group capabilities
CRG as a CRO has extensive experience in conducting Pre-Study Site Selection Visits and choosing the most suitable Sites for successful Clinical Trial execution.
Information on all other Clinical Trial services that we provide could be found at www.crg.global
You can contact us at [email protected]
You Might Also Like
Translation services in clinical trials, project management in clinical trials, investigator meeting and other study meetings.
We use cookies to ensure that you receive the best possible experience on our web-site. If you choose “I ACCEPT” you agree on our use of cookies and data collection processes (see separate “ Internet Privacy Policy ”). If you do not agree please leave our web-site immediately!
Add Your Heading Text Here
Clinical site initiation visit checklist and best practices.

Medha Datar
- March 3, 2023

The clinical site initiation visit is a critical component of the clinical trial start-up process. It involves the CRA visiting the study site to ensure that the site is prepared to conduct the study according to the protocol and Good Clinical Practice (GCP) guidelines. The purpose of the site initiation visit is to confirm that the site has the necessary resources, procedures, and training in place to conduct the study and collect accurate data.

Here are some best practices for conducting a successful site initiation visit:
- Schedule the site initiation visit as early as possible in the study start-up process to allow sufficient time for addressing any issues that may arise.
- Confirm that the site has all the necessary study documents, including the protocol, informed consent form, case report form, and monitoring plan.
- Verify that the site has obtained IRB/EC approval and that all regulatory documents are complete and accurate.
- Ensure that all site staff has completed the required training, including GCP training, and that their CVs are up to date.
- Review the study drug or device management plan and confirm that the site has procedures in place for managing adverse events and protocol deviations.
- Explain the monitoring process to the site staff and discuss the CRA’s role in monitoring the study.
- Confirm that the site has a plan for managing subject enrollment and explain the subject screening and recruitment process.
- Review the case report form with the site staff and explain how to complete it accurately and completely.
- Discuss the communication plan between the site staff and the sponsor/CRO, including how to report issues and the frequency and format of study updates.
- Verify that the site has procedures in place for data management and document retention.
Here is a sample clinical trial initiation visit checklist for a Clinical Research Associate (CRA):
By following these best practices and checklists, the CRA can help ensure that the study is conducted according to the protocol and GCP guidelines and that high-quality data is collected. The site initiation visit is an important opportunity to establish a good working relationship with the site staff and to identify any issues that may need to be addressed before the study begins.
Cloudbyz Unified Clinical Trial Management (CTMS) is a comprehensive, integrated solution to streamline clinical trial operations. Built on the Salesforce cloud platform, our CTMS provides real-time visibility and analytics across study planning, budgeting, start-up, study management, and close-out. Cloudbyz CTMS can help you achieve greater efficiency, compliance, and quality in your clinical operations with features like automated workflows, centralized data management, and seamless collaboration. Contact us today to learn how Cloudbyz CTMS can help your organization optimize its clinical trial management processes.
To know more about the Cloudbyz Unified Clinical Trial Management Solution contact [email protected]
Request a demo specialized to your need.
Subscribe to our weekly newsletter

At Cloudbyz, our mission is to empower our clients to achieve their business goals by delivering innovative, scalable, and intuitive cloud-based solutions that enable them to streamline their operations, maximize efficiency, and drive growth. We strive to be a trusted partner, dedicated to providing exceptional service, exceptional products, and unparalleled support, while fostering a culture of innovation, collaboration, and excellence in everything we do.
Subscribe to our newsletter
CTMS CTBM eTMF EDC RTSM Safety & PV PPM
Business Consulting Services Professional Services Partners CRO Program
Blog e-Books & Brochures Videos Whitepapers News Events
About us Careers Sustainability FAQ Terms & Conditions Privacy Policy Compliance
© 2024 Cloudbyz
My Account / Create Account
Forgot My Password
PracticeMatch Physician Articles
What to expect from your site qualification visit: last minute prep tips.
Site Qualification visits are an essential component of the clinical trials site selection process. This visit allows both you and the trial's Sponsor to learn more about each other and ascertain if the study is a good fit. It is also your chance to show that you and your facility can support the many regulatory and performance requirements that each clinical study demands.
Do you know what to expect during your site visit? Learn more about how you can prepare with these easy tips:

Preparing for the Sponsor
Assessing the suitability of your staff and site is a top priority for Sponsors during a site visit. Sponsor representatives will review the qualifications and background of all participating physicians and staff at your clinical site. They will also assess whether you have sufficient training, time and resources to fulfill the clinical study's requirements.
Prepare for these reviews by:
Having updated and comprehensive CV's on hand for the Sponsor to review for key study personnel. It is important to emphasize any prior clinical trial work or publications. This can also include experience gained while in Residency training. Ensure that you also have copies of applicable medical licenses on hand for review during and following the site qualification visit.
Holding a group protocol review meeting with your staff prior to the site qualification visit. Be sure to include all study staff and discuss the protocol, IRB requirements, SOPs, and any required training in depth. Try to brainstorm any issues that may arise while trying to execute the protocol, as this can help generate points that may need clarification during the visit. Sponsors are looking for experienced, efficient staff members who are engaged and will make their study a priority.
Ensuring you have a recruitment strategy and enrollment target timeline ready ahead of time to share with the Sponsor. Sponsors want to know that you will be able to meet their recruitment goals, so it is key to show that you are prepared with a strategy and have contingency recruitment plans.
Preparing for the Site Qualification Facility Inspection
An important part of any site qualification visit involves inspecting available clinical and storage space. Clinical trials require dedicated areas for study administration, examinations, drug and data storage. Sponsors will assess whether you have the necessary equipment and space to execute their study requirements.
Be sure that all designated areas are used exclusively for their assigned purposes. Prior to inspection, ensure that all areas are clean and have only applicable items stored in the area.
Ensure all applicable laboratory and medical licenses are up to date prior to the start of the site qualification visit.
Check that your facility has met the necessary security requirements in regard to drug storage, data security and access.
Your session is about to expire
Site initiation visit (siv): clinical trial basics, what is an siv in clinical research, siv definition: site initiation visit.
An SIV (clinical trial site initiation visit) is a preliminary inspection of the trial site by the sponsor before the enrollment and screening process begins at that site. It is generally conducted by a monitor or clinical research associate (CRA), who reviews all aspects of the trial with the site staff, including going through protocol documents and conducting any necessary staff training.[ 1 ],[ 2 ]
Also known as a study start-up visit, the sponsor can only request an SIV after the site has been selected and formal agreements such as the CTA have been signed.
What is the purpose of an SIV?
Clinical trial SIVs are necessary to ensure that all personnel of a given site who will be involved in the clinical trial, such as investigators and study staff, thoroughly understand the trial protocol and are trained appropriately so as to handle their role and responsibilities. Furthermore, the site initiation visit has the aim of ensuring the trial site is operationally ready, with working infrastructure, tools, and any study materials needed.[ 1 ]
Given the scope of the SIV, clinical trial sponsors should schedule this visit well before enrollment so that there is plenty of time to comprehensively inspect all relevant processes, and to conduct further training or rectify any issues, if necessary.
Can the SIV be conducted before IRB approval?
IRB approval is generally necessary before the SIV is carried out. Clinical trial sponsors should select sites that are compliant with all applicable regulatory requirements, and after the site receives IRB approval for the research, the sponsor can conduct the SIV.
SIV checklist for thorough site initiation visits
Given the importance of the SIV, clinical trial sponsors should make the most of this inspection visit by coming fully prepared with a detailed checklist of what is to be confirmed during the SIV.
Clinical trial sites might receive a copy of this checklist so they can ensure that all relevant staff are present for the visit. Specific tasks to include in the SIV checklist include the following:[ 1 ],[ 2 ],[ 3 ],[ 4 ]
- Discussing the clinical trial’s objectives with study staff
- Educating the research team on Good Clinical Practices
- Reviewing the operation schedule for the protocol
- Discussing the enrollment and screening process, including clarifying the inclusion and exclusion criteria
- Reviewing the informed consent documents and procedure
- Clarifying procedures for storing, dispensing, and managing the investigational product (IP)
- Checking inventory for all required medical supplies and equipment
- Ensuring secure access to all digital platforms, i.e., correct usernames and passwords
- Touring the clinical trial site to ensure facilities are in proper condition
- Reviewing and discussing all clinical trial documentation, such as forms, surveys, SOPs, etc.
- Reviewing the data management system and any other technological solutions forming part of the site’s or sponsor’s workflow
- Ensuring that site staff are up to date on training and understand how to maintain essential documentation
- Reviewing the site/trial budget financial protocols, including any processes related to compensating trial participants
- Verifying and testing reporting procedures possible adverse events
- Leaving room for an open discussion of any specific concerns that trial staff may have
This checklist provides basic guidelines only, and should be built upon and customized for each individual study according to risk areas and specific protocols.
Other Trials to Consider
Modified Atkins diet
Administration of bms-986460, family-based mental health navigation, high dose gsk4532990, fecal microbiota transplant (fmt) oral capsules, capivasertib, milk thistle (mt), perivascular dexamethasone, popular categories.
Colon Cancer Clinical Trials 2024
Cannabis Clinical Trials 2024
Prostate Clinical Trials 2024
Zika Virus Clinical Trials 2024
Paid Clinical Trials in Milwaukee, WI
Ofev Clinical Trials
Smoking Cessation Clinical Trials 2024
Scleroderma Clinical Trials
Semantic Dementia Clinical Trials 2023
Craniopharyngioma Clinical Trials 2023
Popular guides.

Optimising the Site Selection Process
Assessment of investigator motivation, benjamin quartley, associate director feasibility and patient recruitment, clinical development services covance, uk.
Identifying sites which enroll in line with expectations represents a major challenge in clinical research. This article discusses strategies to address this challenge and explores the golden site profile concept.
According to some estimates, up to 30 per cent of Investigators participating in a trial may recruit no patients. When considering this statistic, it is important to remember that every site—also those that have not recruited to target—has been through a process of selection. Whilst this process may determine whether a site can provide suitable staff and facilities in accordance with regulatory guidelines, it is evident that the site selection process is not foolproof in assessing whether or not a site will in fact recruit patients as anticipated, or at all.
This article evaluates a number of pragmatic approaches to more effectively identify suitable sites by proactively weeding out the types of surprises that—with the 20 / 20 vision of hindsight—were not so very surprising. We move beyond the basics of the site selection visit and consider the process as both a strategic and tactical challenge focussing on three key factors:
- The impact of company processes in setting the tone for success or failure
- Identifying the less obvious but crucial characteristics of successful sites
- The importance of investigator motivation.
The golden site profile Looking to the investigators, prior experience and personal relationships often form the basis for selection. Given the increasing volume of competing trials, experienced investigators are in great demand. However, they may not offer the certainty of delivery they once did. Data also suggests that the number of investigators active for only one year in clinical trials is increasing. Taken together, the necessity to work with new investigators becomes apparent. In such circumstances, it is important to extrapolate from previous experience to identify the indirect and less obvious characteristics associated with good sites—characteristics that may not be apparent at the individual site selection visit. For instance, an analysis of stroke studies conducted at over 400 sites globally for over a five-year period showed that the top decile of investigators (in terms of recruitment) displayed the following characteristics:
- Located in countries / cities with an older population
- Practicing in a large or general hospital
- Located in more densely populated cities / countries
- Specialising in neurology with an emphasis on geriatrics.
Whilst perhaps not surprising, these findings allow the conclusion that the successful recruitment of patients in such studies is linked to the ability to identify investigators who fulfill this specific set of criteria.
Assessing investigator motivation There is, of course, only so much that can be predicted from an investigator survey process and analysis of past data. Assuming the investigators are located in the same country, have access to similar patient populations, similar facilities and staff, why do we continue to see a broad range in recruitment performance across sites that appear similar or at least equivalent? To help explain this, we turn to two of the less tangible aspects of successful sites—investigator interest and motivation. For most investigators clinical research is only a small part of their practice, compared to the dominant obligation of patient care. Moreover, the investigators continually face time constraints and have to prioritise their time. In this context the importance of investigator motivation is evident. When considering motivation we should first address why investigators make the decision to take part in clinical trials? Reasons vary and are specific to each individual investigator, but generally include potential benefits to patient, scientific or medical interest and financial benefit. In today’s world of health economics, trials may also be a conduit not only to the test drug, but also the comparator drug that may already be on the market but cost-prohibitive to many patients. Regardless of what drives their motivation, it is necessary to confirm that investigators participating in a trial possess this key ingredient if the current cycle of delayed studies is to be broken. With experience, it is possible for the site selection staff to get a general impression of how motivated an investigator is. To more accurately assess investigator motivation, consideration should be given to the following practical approaches:
Strategic partnering – Depending on the healthcare system, institutions may have a Research & Development office that has worked with investigators over a number of years. By meeting only with the individual investigator and maintaining a transactional relationship, instead of collaborating also with centralised R&D functions, we may continue to select investigators in a relatively blinded fashion. However, developing shared objectives and working collaboratively with such central functions can add great value and prevent inappropriate investigators from being approached.
Asking the right people – It’s no surprise that the research nurse or other members of the team running trials day-to-day will be able to give a more accurate view of the practical implementation of a trial than the investigators themselves. Similarly, an investigator may not be aware whether support functions, such as the radiography department, can provide the volume and frequency of scans required. Such logistical challenges often are the hidden reason for study delays and should always be assessed directly and proactively with the individuals responsible.
Asking the right questions – Site selection visits that start with a protocol presentation followed by an invitation for the investigator to ask questions in fact veil how much time the investigator spent reading the protocol in advance of the visit. Instead, starting the visit by simply asking the investigator their opinion of the protocol is a good indication of their level of interest and motivation.
In summary, a site selection visit that ensures facilities and qualified staff are in place is a relatively straightforward process. However, determining if a site will actually recruit patients into the trial is another matter entirely. In order to make the process more effective and successful, we need to start by creating a culture and company processes that engender ownership and encourage the selection of the right sites. On a day-to-day basis, application of the assessment approaches explored in this article provides a greater probability of selecting investigators that will recruit as anticipated.
Do we as an industry set the scene for success or failure? Before considering the investigators themselves, we should challenge ourselves as to our own role, as an industry, in building an environment that supports successful site selection.
- How often does a site selection visit result in the investigator not participating in the study? With up to 30 per cent of site not recruiting any patients, should we not also expect 30 per cent of the sites we visit to not go forward? Naturally, it is not this simple, but with relatively few sites dropping out at this stage it should be considered whether the balance is in the right place.
- How often are investigators re-selected who have previously taken part in trials but not recruited any patients? Again, this is not straightforward given the various reasons why an investigator may not have recruited patients to a previous trial, or why the investigator may be selected for continued trial participation. However, we should at least have a realistic view of the investigator’s likely contribution and account for this in study planning.
- Are the individuals who select investigators accountable for the subsequent performance of the investigators? One team of people may be responsible solely to select a set number of investigators from a limited pool, and may be facing considerable time pressure to do so. As such, their chief concern will be to meet the immediate commitment in terms of the number of sites. This can create a disconnect between site selection and the actual trial conduct, which may be exacerbated when the team managing the sites throughout the study receives such a list of investigators they did not select themselves. If not appropriately and proactively managed, this process allows for a lack of ownership and responsibility for site performance.
- Do the individuals responsible to select the investigators have the required experience to assess some of the more complex factors, for instance investigator motivation? CRAs may be trained to select investigators in line with ICH and the protocol. However, is the site selection staff trained to effectively assess the less tangible aspects of what makes for a successful site? Moreover, is the staff in a position to reject investigators they do not consider suitable?
- There simply may not be sufficient sites that meet all the criteria. This is an increasing problem in many therapeutic areas, notably oncology. Consider whether we are being too conservative in terms of investing in new investigators in emerging markets and facilitating their trial participation. Are we actually taking greater risk by not being more inclusive in this regard?
Selecting investigators who truly deliver is an issue that goes deep into the operations of a company—and this is ultimately where it is within our own control to set the tone for success or failure.
For additional information on methods to identify the golden site profile, please refer to the article ?Data Based Predictions? by Michelle Jones, MSc, CStat and Stephen Jones, MSc, CStat, published in Applied Clinical Trials magazine, March 2008, vol 17, no 3.

Benjamin Quartley started his career working as a CRA in the Pharmaceutical Industry. Recognising the challenges presented in patient recruitment he joined a company established to support Investigators in the delivery of clinical trials, where he gained an insight into the Investigators world across eight years. Ben has now taken this experience back to the Industry and works for Covance in Feasibility and Patient Recruitment.

- Open access
- Published: 11 December 2019
Criteria for site selection in industry-sponsored clinical trials: a survey among decision-makers in biopharmaceutical companies and clinical research organizations
- Tilde Dombernowsky ORCID: orcid.org/0000-0001-8930-1341 1 ,
- Merete Haedersdal 1 ,
- Ulrik Lassen 2 &
- Simon Francis Thomsen 1 , 3
Trials volume 20 , Article number: 708 ( 2019 ) Cite this article
17k Accesses
20 Citations
2 Altmetric
Metrics details
Knowledge of what the pharmaceutical industry emphasizes when assessing trial sites during site selection is sparse. A better understanding of this issue can improve the collaboration on clinical trials and increase knowledge of how to attract and retain industry-sponsored trials. Accordingly, we investigated which site-related qualities multinational biopharmaceutical companies and clinical research organizations (CROs) find most important during site selection.
An online survey among decision-makers for trial site selection in the Nordic countries employed at multinational biopharmaceutical companies and CROs was conducted. The respondents’ experiences with and perceptions of site selection were addressed to evaluate the relative importance of site-related qualities. We included up to four respondents per company, representing different geographic regions. Descriptive statistics were used to summarize findings.
Of 49 eligible companies, 20 biopharmaceutical companies and 23 CROs participated. In total, 83 responses were analyzed (estimated response rate 78%). A relative importance of site-related qualities was identified: For example, 88% (binomial 95% confidence interval [CI] ±7%) preferred reaching enrollment goals at trial sites in their region 10% quicker rather than cutting the costs at all sites by 20%. Likewise, 42% (CI ±11%) of the respondents preferred that trial sites were best at having the first patients ready for inclusion right after site initiation visit compared to having good data entry, documentation, and reporting practice (25% [CI ±9%]), easily reachable site personnel and backup (23% [CI ±9%]), fast contractual procedure times (6% [CI ±5%]), a key opinion leader associated with the site (3% [CI ±4%]), and updated equipment and facilities (1% [CI ±2%]). In total, 75% [CI ±9%] agreed that their company would be interested in cooperating with an inexperienced trial site if the site had access to a large patient population and 52% [CI ±11%] had experienced that their company selected an inexperienced trial site in favor of an experienced site due to a higher level of interest and commitment.
Conclusions
This study indicates that recruitment-related factors are pivotal to the pharmaceutical industry when assessing trial sites during site selection. Data quality-related factors seem highly valued especially in early phase trials whereas costs and investigator’s publication track record are less important. Experience in conducting clinical trials is not imperative. However, this applies primarily to late phase trials.
Peer Review reports
When the pharmaceutical industry assesses potential trial sites during trial site selection, multiple aspects are considered. Factors such as patient population availability, resources at the site, and data collection procedures are evaluated. Likewise, site personnel-related qualities such as interest and commitment, communicative skills, and experience in conducting clinical trials are taken into account. Today, site management is often handled by clinical research organizations (CROs) as many clinical trials are outsourced [ 1 ]. Consequently, CROs play a pivotal role during site selection alongside the affiliates of biopharmaceutical companies.
Knowledge of what the pharmaceutical industry emphasizes when selecting European trial sites is sparse; to our knowledge, only two publicly available studies have investigated this [ 2 , 3 ]. They indicate that recruitment-related factors are pivotal whereas costs are less important. Moreover, they suggest that experience in conducting clinical trials is not imperative.
A better understanding of what the pharmaceutical industry emphasizes when assessing trial sites during site selection can improve the collaboration and performance in clinical trials, ultimately leading to improved medical care. Moreover, a better understanding of this issue can extend knowledge of how trial sites can attract and retain industry-sponsored trials. Accordingly, we conducted a survey among decision-makers for trial site selection in biopharmaceutical companies and CROs to further explore this area.
The aim of this study was to investigate which site-related qualities multinational biopharmaceutical companies and CROs find most important during site selection and while running clinical trials in the Nordic countries. In continuation of the findings by Gehring et al. [ 3 ] and findings we made in an interview study conducted in 2016 [ 2 ], we particularly focused on recruitment-related factors, costs, and experience in conducting clinical trials. Three main assumptions generated from this previous research were explored:
Biopharmaceutical companies and CROs find that recruitment-related factors (i.e. patient population availability, timely patient recruitment, and startup time) are the most important factors during site selection and while running clinical trials;
Experience in conducting clinical trials is not imperative to biopharmaceutical companies and CROs when selecting clinical trial sites;
The costs of running a clinical trial are secondary to biopharmaceutical companies and CROs if trial sites recruit the patients agreed upon in a timely matter.
Identification of companies and respondents
Our recruitment strategy focused on personal contacts to ensure that relevant companies and respondents were included. First, we identified companies involved in trial site selection in one or more Nordic countries. Thereafter, we identified suitable respondents within each company. Figure 1 illustrates the company selection process.

Flow chart showing the identification of eligible companies * The Danish Association of the Pharmaceutical Industry, The Swedish Association of the Pharmaceutical Industry, The association for the pharmaceutical industry in Norway, Pharma Industry Finland, The trade association and forum for clinical research organizations active in Sweden, The CRO network of Trial Nation Denmark. # CRO clinical research organization
The following inclusion criteria for the companies were set:
Multinational biopharmaceutical company or CRO;
Conducted clinical trials in one or more Nordic countries;
The affiliate(s) / local office(s) of the company were involved in trial site selection in one or more Nordic countries;
Member of one of the following organizations: The Danish Association of the Pharmaceutical Industry; The Swedish Association of the Pharmaceutical Industry; the association for the pharmaceutical industry in Norway; Pharma Industry Finland; the trade association and forum for clinical research organizations active in Sweden; and the CRO network of Trial Nation Denmark.
The following inclusion criteria for the respondents were set:
Employed at one of the included companies at a Nordic affiliate / local office;
Decision-maker for trial site selection in one or more Nordic countries or involved in the recommendation of trial sites to the sponsor(s).
Using the trial registry ClinicalTrials.gov [ 4 ], we estimated that the member companies of the included organizations sponsor or are collaborators in 79% of all industry-sponsored clinical trials conducted in the Nordic countries (Additional file 1 ). Consequently, we believe that we included the majority of companies involved in trial site selection in the Nordic countries.
Eligible companies and respondents were identified through contact with the Nordic and European affiliate(s) or office(s) by email or phone. A contact person—who in most cases was also a respondent—was sent a link to the online survey and forwarded the link to other eligible participants within the company. We included up to four respondents per company, representing different geographic regions (Denmark, Finland, Norway, and Sweden) as decision-makers for trial site selection employed at the same company may have different perceptions on site selection depending on the region in which they operate. Respondents were recruited continuously during the whole survey response period from 8 May to 8 October 2018. The period was expanded for 1.5 months due to the summer holidays. Because of the recruitment design, the identity of most respondents was known to the authors. However, the respondents were assured that the results would be published without any disclosure of their identity. No remuneration was provided but a summary of the survey results before publication was offered. Additional information on the recruitment process and survey distribution is displayed in the Additional file 1 .
Content of the survey
The survey was a web-based questionnaire addressing the respondents’ perceptions of factors that influence trial site selection in the Nordic countries. Some items aimed at the respondents’ personal opinions, whereas others aimed at the overall opinion of their company. The survey consisted of a background information section followed by three main sections and was completed in 10–15 min using the SurveyXact online platform [ 5 ]. The items were presented primarily in Likert scale, single response, and ranking format. In the first section, respondents were asked to indicate their level of agreement with different statements using a five-point scale (strongly agree, agree, undecided, disagree, strongly disagree). In the second section, the respondents’ own experiences with site selection at their company were addressed using primarily single response questions; in the last section, ranking questions were used to evaluate which site-related qualities are the most important in different situations. To avoid missing data, all questions had to be answered before continuing to the next section. To minimize response bias, response categories of the ranking questions were randomly ordered for each respondent individually.
Due to differences in the organizational structure and function of the companies, some items had to be differently formulated depending on the respondent being employed at a biopharmaceutical company or a CRO. Therefore, the two respondent groups received a different questionnaire, although the content was almost identical. For example, during pretesting, CRO respondents stressed that CROs are recommending trial sites to the sponsor and not selecting trial sites. Therefore, the word selected was replaced with recommended in relevant items as illustrated in Table 1 . We believe that the different wording of the items ensured a homogeneous interpretation of each item across the two respondent groups, still making it possible to evaluate the items as one. However, two items were evaluated separately as the wording differed markedly (Table 1 , question 5 and 6; Fig. 2 , questions 2 and 3). The full survey for biopharmaceutical and CRO respondents, respectively, are displayed in Additional file 1 .
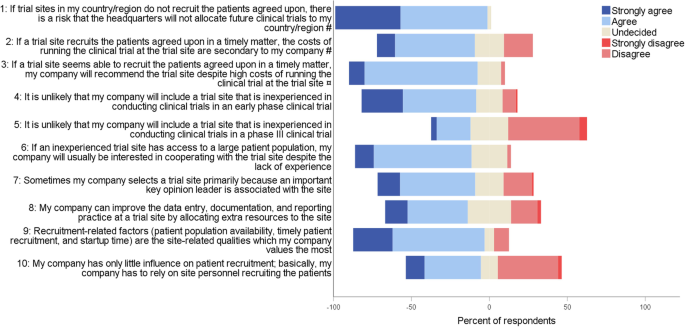
Levels of agreement with statements about trial site selection in the Nordic countries # This question applied to only biopharmaceutical respondents ( n = 43) ¤ This question applied to only CRO respondents ( n = 40). CRO clinical research organization
Two items in the background section served to ensure that the respondent and the company were indeed decision-makers for trial site selection. If this was not confirmed, the respondent was excluded. Further, the respondent’s company email address was requested to verify that the response came from a relevant person, to determine which company was involved, and to avoid duplicate responses.
Development and validation of the survey
The development of the survey was based on a previous interview study including employees involved in trial allocation at multinational biopharmaceutical companies [ 2 ] and other literature within this field [ 3 , 6 , 7 , 8 , 9 , 10 , 11 , 12 ]. First, we developed an exhaustive list of site-related qualities that the pharmaceutical industry potentially considers during site selection. Subsequently, the items of the survey were constructed, repeatedly reviewing the list, and the three main assumptions that we aimed to investigate. The design and content of the survey were discussed among the authors and iteratively with relevant clinical trial stakeholders and two statisticians. The initial items were scrutinized to mitigate ambiguity and identify concepts that needed to be validated during pretesting, such as early phase clinical trial and data quality . These concepts were listed and systematically reviewed during pretesting. The pretesting included 19 potential respondents employed at different companies and was carried out at meetings lasting 45–75 min, using a standardized procedure. Additional information on the development and validation of the survey is displayed in Additional file 1 .
Statistical analysis and sample size considerations
We used descriptive statistics to summarize findings. Binomial 95% confidence intervals (CIs) were calculated using the equation for the normal approximation for the binomial confidence interval: p ± z 1-α/2 √(p (1-p)/n). To evaluate potential differences in responses across the two respondent groups, we compared responses using Chi-squared tests and Fisher’s exact tests. Ranking questions were evaluated by comparing differences in the number of first rankings within each response category across the two respondent groups. As the number of respondents in each group was small, we also considered the true values observed. Data were analyzed using SPSS Version 25. A p value threshold of ≤ 0.05 was considered statistically significant. There were no missing data as all responses were complete. Given the descriptive design and a finite number of respondents, we did not formally estimate a required sample size.
Of the 49 eligible companies, 20 biopharmaceutical companies (83%) and 23 CROs (92%) participated in the survey (Fig. 1 ). The number of decision-makers for trial site selection in the Nordic countries varied between the companies that differed markedly in size and organizational structure. A total of 101 responses were received, of which none were duplicate. Six were partial and all excluded as they were < 20% completed. Further, two were excluded as the respondents reported not to be decision-makers for trial site selection. We received more than one response per Nordic country from four companies. Consequently, 10 responses from these companies were excluded randomly using SPSS. In total, 83 responses were analyzed: 43 from biopharmaceutical companies and 40 from CROs. The average number of respondents per company was 1.9 (standard deviation [SD] 1.1), and the estimated response rate was 78% for both respondent groups (see Additional file 1 ). The respondents’ type of position and level of experience are displayed in Table 2 .
Recruitment-related factors (assumption 1)
In total, 84% (CI ±8%) of the respondents strongly agreed or agreed that recruitment-related factors are the site-related qualities that their company values the most (Fig. 2 , question 9). Likewise, 88% (CI ±7%) preferred reaching enrollment goals at trials sites in their region 10% quicker rather than cutting the costs at all sites by 20% (data not shown). When asked to rank which information about a trial site unknown to their company that the company would find the most valuable, recruitment and retention track record was ranked first by 71% (CI ±10%) of the respondents among the six factors tested (Additional file 1 : Figure S1). Similarly, when the respondents were asked what they would prefer that trial sites were best at, 42% (CI ±11%) ranked having the first patients ready for inclusion right after site initiation visit first (Fig. 3 ).

What decision-makers for trial site selection would prefer that Nordic trial sites were best at* * Respondents ( n = 83) were asked: If you could choose, what would you prefer that trial sites were best at? The six response categories were ranked from one to six, one being the most important. MR mean ranking (of the response category), SD standard deviation
Figure 4 illustrates the ranking of five site-related qualities according to importance during site selection. For early phase trials, having a large patient population available at the site was ranked first by 33% (CI ±10%), whereas it was 54% (CI ±11%) for phase III trials. Two items addressed which of three site-related qualities the clinical operations departments at the affiliates value the most while running an early phase and phase III trial, respectively. Timely patient recruitment was ranked the highest in both cases (57% [CI ±11%] and 59% [CI ±11%], respectively) compared to timely data entry and reporting (10% [CI ±6%] and 12% [CI ±7%], respectively) and no critical or major findings at the site during the trial (33% [CI ±10%] and 29% [CI ±10%], respectively) (Additional file 1 : Figure S2). As illustrated by Fig. 5 , overestimation of the available study population and insufficient site personnel resources or backup at the site are the site-related qualities that most often cause delay in patient recruitment at Nordic trial sites according to the respondents.
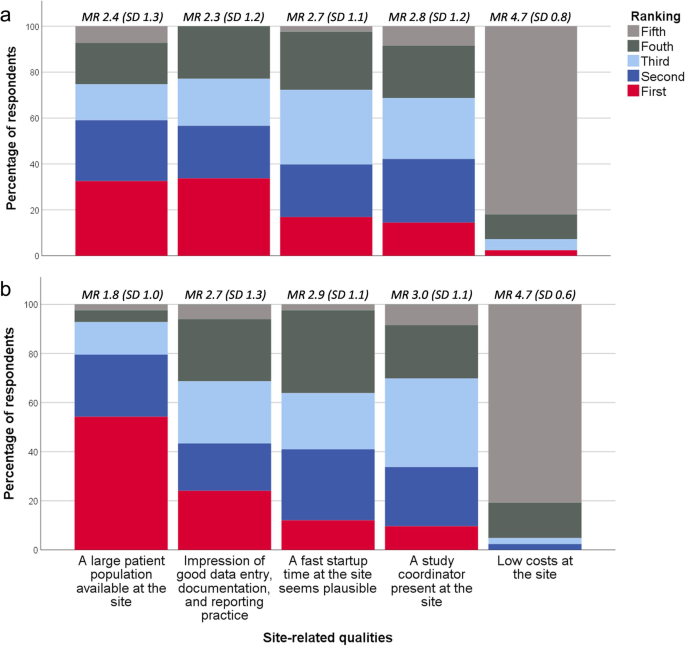
Relative importance of site-related qualities for early phase ( a ) and phase III trials ( b )* * Respondents ( n = 83) were asked which of five site-related qualities their company finds the most important during site selection for an early phase clinical trial and phase III clinical trial, respectively. The five response categories were ranked from one to five, one being the most important. MR mean ranking (of the response category), SD standard deviation

Site-related factors that do most often cause delay in patient recruitment at Nordic trial sites* * Respondents ( n = 83) were asked to choose among 12 site-related factors the four factors they believe most often cause delay in patient recruitment at the Nordic trial sites that their company cooperates with. Only factors that trial sites influence were included
Two items addressed which factors the headquarters of biopharmaceutical companies find the most important when evaluating the affiliates’ performance and CROs’ performance, respectively, regarding running clinical trials. For both early phase and phase III trials, timely patient recruitment was ranked first by most respondents (58% [CI ±11%] and 57% [CI ±11%], respectively) compared to high data quality (35% [CI ±10%] and 24% [CI ±9%], respectively), timely data entry and reporting (4% [CI ±4%] and 10% [CI ±6%], respectively), and low costs of running the clinical trial (3% [CI ±4%] and 9% [CI ±6%], respectively) (Additional file 1 : Figure S3).
Experience in conducting clinical trials (assumption 2)
In total, 75% (CI ±9%) strongly agreed or agreed that their company would be interested in cooperating with an inexperienced trial site if the trial site had access to a large patient population (Fig. 2 , question 6). Further, 52% (CI ±11%) had experienced that their company selected an inexperienced trial site in favor of an experienced site due to a higher level of interest and commitment (Table 1 , question 1). In contrast, 74% (CI ±9%) of the respondents strongly agreed or agreed that it is un likely that their company would include an inexperienced trial site for an early phase trial; for phase III trials, it was only 25% (CI ±9%) (Fig. 2 , questions 4 and 5).
Respondents were asked to rank which of three site personnel-related qualities their company finds the most important during site selection: Experience in conducting clinical trials was ranked first by 59% (CI ±11%) for early phase trials and 46% (CI ±11%) for phase III trials, whereas impression of a high level of interest and commitment was ranked first by 33% (CI ±10%) and 48% (CI ±11%), respectively (Fig. 6 ). Most respondents believed that if trial site personnel seek out stakeholders at biopharmaceutical companies at conferences displaying a site profile form and track record, the companies would consider including the trial site in future clinical trials: yes definitely (24% [CI ±9%]); yes maybe (70% [CI ±10%]); and no (6% [CI ±5%]).
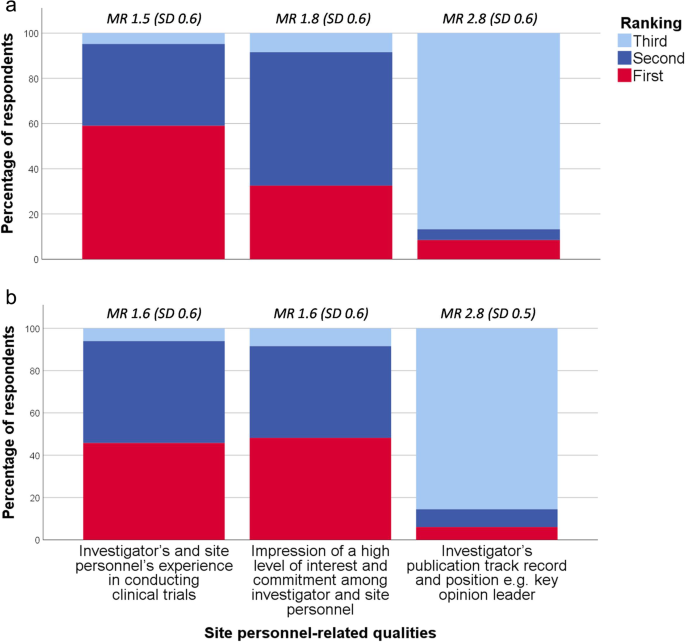
Relative importance of site personnel-related qualities for early phase ( a ) and phase III trials ( b )* * Respondents ( n = 83) were asked which of three site personnel-related qualities their company finds the most important during site selection for an early phase clinical trial and phase III clinical trial, respectively. The three response categories were ranked from one to three, one being the most important. MR mean ranking (of the response category), SD standard deviation
Costs (assumption 3)
Most respondents strongly agreed or agreed that the costs of running a clinical trial at a trial site are secondary if the site recruits the patients agreed upon in a timely matter (Fig. 2 , questions 2 and 3). Likewise, when asked which site information is the most valuable to their company, prices of all trial-related services was ranked the lowest alongside data on potential investigators’ publication track record and job position (Additional file 1 : Figure S1). Similarly, low costs at the site was ranked lowest among five site-related qualities regarding their importance during site selection (Fig. 4 ). For both early phase and phase III trials, low costs of running the clinical trial was ranked lowest when considering which factors the headquarters find the most important when evaluating the affiliates and CROs (ranked fourth by 70% [CI ±10%] and 63% [CI ±10%], respectively) (Additional file 1 : Figure S3).
Sensitivity analysis
Overall, the response pattern was similar across the two respondent groups. However, more biopharmaceutical than CRO respondents preferred trial sites having the first patients ready for inclusion right after site initiation visit (ranked first by 56% [CI ±15%] and 28% [CI ±14%], respectively ( p = 0.014)) rather than sites having good data entry, documentation, and reporting practice (19% [CI ±12%] and 33% [CI ±15%], respectively ( p = 0.207)). Moreover, notable differences occurred regarding which factors the headquarters of biopharmaceutical companies value the most when evaluating the affiliates’ and CROs’ performance in relation to running clinical trials. Timely patient recruitment was ranked first by more biopharmaceutical than CRO respondents for both early phase and phase III clinical trials (65% [CI ±14%] vs 50% [CI ±15%] for early phase trials [ p = 0.187]; and 74% [CI ±13%] vs 38% [CI ±15%] for phase III trials [ p = 0.001]). Conversely, low costs of running the clinical trial was ranked first by more CRO respondents (8% [CI ±8%] vs 0% of biopharmaceutical respondents for early phase trials [ p = 0.108]; and 18% [CI ±12%] vs 2% [CI ±5%] for phase III trials [ p = 0.026]).
In this survey that investigated which site-related qualities the pharmaceutical industry values the most during site selection in the Nordic countries, recruitment-related factors were strongly emphasized, whereas costs and investigator’s publication track record generally had low priority. Data quality-related factors and experience in conducting clinical trials were strongly emphasized in early phase trials, whereas experience was less emphasized in phase III trials.
Recruitment-related factors were highly emphasized throughout the survey for both early phase and phase III clinical trials. This gives weight to the supposition that access to the relevant patient population, a fast startup time, and timely recruitment are among the most important factors when the pharmaceutical industry evaluates trial sites during site selection. Nevertheless, the survey results also indicate that other qualities are sometimes more important. For example, we found that 61% of the respondents had experienced that their company selected a trial site which delivered an insufficient recruitment in prior trials, because a key opinion leader was associated with the site (Table 1 , question 3).
According to the respondents, one of the main reasons for insufficient recruitment at Nordic trial sites is overestimation of the available study population at the site, when considering factors that trial sites influence. This concurs with findings in our previous interview study in which the participants reported that they often find the investigators’ recruitment projections over-optimistic [ 2 ]. Consequently, their company routinely marks down these. This has also been reported by others [ 13 , 14 ]. Trial sites should take this into consideration and strive to make accurate recruitment projections by carefully considering aspects of the current trial rather than following “gut intuition” or replicating estimations from prior similar trials. That said, the sponsors are also responsible for inaccurate recruitment projections. First, the investigators typically do not have full protocol information when requested to estimate the number of participants the trial site can recruit and the information given by the sponsor changes over time. Second, the response deadline is short, limiting time for a thorough assessment. Third, trial sites are not economically compensated for the time spent which impedes investigators’ motivation to make thorough estimations. As recruitment projections strongly influence study timelines, accurate projections should be of high priority among both trial sites and sponsors to mitigate trial extensions and failure.
Data quality-related factors were generally emphasized less than recruitment-related factors in this survey. One explanation could be that sufficient patient recruitment is crucial to the success of a trial whereas good data quality is not. Another explanation could be that the companies have only little influence on recruitment whereas they can more easily ensure sufficient data quality by allocating extra resources to monitoring and training at the site. However, the results do not confirm this assumption, as responses were ambiguous in this matter (Fig. 2 , questions 8 and 10). In our previous interview study, only half of the participants spontaneously mentioned data quality-related factors as important [ 2 ]. Moreover, like in this survey, some believed that the headquarters of their company did not value data quality as high as timely patient recruitment. However, when asked, the participants stressed that they find high data quality indispensable. Possibly, these findings reflect that high data quality is essential; however, as there are no data without participants, recruitment is emphasized more than data quality during site selection.
Interestingly, the survey results suggest that biopharmaceutical companies and CROs are interested in collaborating with inexperienced trial sites if they have access to the relevant patient population and show interest and commitment. Moreover, interest and commitment is supposedly as important as experience in conducting clinical trials during selection for phase III trials. This concurs with findings by Gering et al. [ 3 ] who asked 341 different clinical trial stakeholders to divide 100 points across five investigator-related qualities when selecting trial sites for a phase III/IV trial ( investigator recruitment/retention track record , experience in previous trials , interest , concurrent workload , and publication track record ). They found that interest was rated as high as experience in previous trials (mean 22.4 [SD 13.4] and 22.7 [SD 12.0], respectively). In accordance with our study, investigator’s publication track record was least important. The Danish Association of the Pharmaceutical Industry (LIF DK) has also found that commitment is important during site selection. In 2015, LIF DK asked their member companies to describe which site-related qualities they emphasize for early phase trials (personal correspondence with LIF DK). It was stressed that site personnel’s expertise, dedication, and availability are particularly important. Additionally, it was mentioned that the member companies often cooperate with the same preferred trial sites in early phase trials which makes it challenging for inexperienced trial sites to gain cooperation on early phase trials. This is in line with the results of our survey and previous interview study [ 2 ] that propose that experience in conducting clinical trials is more important during selection for early phase that late phase trials. This is unsurprising, as early phase trials are usually operationally complex and demand a high level of expertise.
Our results clearly indicate that costs are less important than other factors during site selection, which concurs with previous findings [ 2 , 3 , 8 ]. Nonetheless, this does not necessarily mean that costs are unimportant; costs may play an essential role during country selection, thereby indirectly influencing site selection. Interestingly, our results suggest that costs are of higher influence when the headquarters evaluate the performance of CROs than the performance of their own affiliates. Given the fact that CROs are external partners, this is unsurprising.
We believe that trial sites that already meet the site personnel and facilities requirements necessary to be considered for selection may benefit from emphasizing three aspects in particular during site selection: (1) a thorough and sound assessment of the patient population available at the site; (2) a high level of interest and commitment among site personnel; and (3) a good data entry, documentation, and reporting practice. Further, trial sites that wish to attract industry-sponsored clinical trials will possibly benefit from seeking out stakeholders from the pharmaceutical industry displaying a site profile form and track record. Trial sites should keep in mind that the recruitment performance at one trial site influences the allocation of trials to all sites in the region as the headquarters of biopharmaceutical companies may not allocate future trials to a region delivering an insufficient patient recruitment.
Strengths and limitations
We believe that this study displays interesting and credible findings. The internal validity of the study is high as the survey was thoroughly constructed and pretested; the respondents were individuals with good reading comprehension who use similar terminology. However, the study has limitations. The number of respondents included in this survey was low; fewer companies than expected were involved in trial site selection in the Nordic countries and several companies had only one primary decision-maker for all Nordic countries. Nevertheless, the respondents were highly representative of the population that we wanted to investigate and the response rate was high. Moreover, the survey included most companies involved in trial site selection in the Nordic countries. To ensure sufficient survey completion, we had to strictly limit the completion time. Consequently, relevant items were omitted which limits the interpretation of the results. Additionally, some site-related qualities were not evaluated. For example, a company’s prior experience with a site is important when selecting trial sites [ 13 , 15 ]. However, the importance of a good working relationship with the site or site personnel having the right mindset is difficult to evaluate in a quantitative setting. We suspected that all qualities would be rated as highly important if they were simply rated individually. Instead we used ranking questions to assess the relative importance of the site-related qualities. However, this method may lead to more “satisficing” behaviour as rank ordering potential responses is a higher level cognitive task.
The present study indicates that recruitment-related factors are pivotal to the pharmaceutical industry when assessing trial sites during site selection. Data quality-related factors seem highly valued especially in early phase trials, whereas costs and investigator’s publication track record are generally less important. Experience in conducting clinical trials is not imperative; biopharmaceutical companies and CROs are supposedly interested in cooperating with inexperienced trial sites if they have access to the relevant patient population. However, this applies primarily to late phase trials.
This is one of the first studies investigating which qualities at a trial site the pharmaceutical industry values the most when deciding which trial sites to preferably cooperate with. Hopefully, the findings will contribute to improved collaboration and performance in industry-sponsored clinical trials and help trial sites gain involvement in these trials. In future studies, it would be highly relevant to explore the investigators’ and trial sites’ perspective. For example, little is known about what motivates investigators and trial sites to conduct clinical trials, and what they emphasize when cooperating with the pharmaceutical industry. This area should be further investigated as it is key to understanding how countries and trial sites can attract and retain industry-sponsored clinical trials as well as how to better the cooperation and performance in clinical trials.
Availability of data and materials
The full survey for biopharmaceutical and CRO respondents, respectively, are displayed in Additional file 1 . The dataset analyzed during the current study are not publicly available to ensure anonymity of the survey participants but are available from the corresponding author on reasonable request.
Abbreviations
- Clinical research organizations
The Danish Association of the Pharmaceutical Industry
Standard deviation
Research and Markets. The new trends of global clinical development outsourcing market. Los Angeles: Business Wire; 2015. https://www.businesswire.com/news/home/20150130005621/en/Research-Markets-2015-Trends-Global-Clinical-Development #. Accessed Dec 2018.
Dombernowsky T, Haedersdal M, Lassen U, Thomsen SF. Clinical trial allocation in multinational pharmaceutical companies - a qualitative study on influential factors. Pharmacol Res Perspect. 2017;5(3):e00317.
Article Google Scholar
Gehring M, Taylor RS, Mellody M, Casteels B, Piazzi A, Gensini G, et al. Factors influencing clinical trial site selection in Europe: the Survey of Attitudes towards Trial sites in Europe (the SAT-EU Study). BMJ Open. 2013;3(11):e002957.
U.S. National Library of Medicine. ClinicalTrials.gov background. 2018. https://clinicaltrials.gov/ct2/about-site/background . Accessed Mar 2018.
Google Scholar
Rambøll Management Consulting. SurveyXact. https://www.surveyxact.dk/ . Accessed 1 Mar 2018.
Chalk K. Site selection - identifying high performing clinical sites: an insider’s insight: Niche Science and Technology; 2017. http://www.niche.org.uk/asset/insider-insight/Insider-Site-Selection.pdf . Accessed Dec 2018
Fischer E. Trial and error - site selection for clinical studies: Pharmaceutical Technology; 2011. https://www.pharmaceutical-technology.com/features/feature130340/ . Accessed Apr 2016
Gossen R. A site’s best strategy for attracting new studies: Rebar Interactive; 2011. http://rebarinteractive.com/patient-recruitment-business-development/ . Accessed June 2016
Harper BD, Zuckerman D. Critical success factors for planning for site selection and patient recruitment planning. BioExecutive Int. 2006;2(6):16–28.
Hurtado-Chong A, Joeris A, Hess D, Blauth M. Improving site selection in clinical studies: a standardised, objective, multistep method and first experience results. BMJ Open. 2017;7:e014796.
Silva A. Selecting study-appropriate clinical sites in 3 steps. Appl Clin Trials. 2018; http://www.appliedclinicaltrialsonline.com/selecting-study-appropriate-clinical-sites-3-steps . Accessed Nov 2018.
Silverman M. 11 considerations in choosing the right clinical trial sites: BioStrategics Consulting; 2011. https://biostrategics.wordpress.com/2011/05/23/11-considerations-in-choosing-the-right-clinical-trial-sites/ . Accessed Jan 2018
Bose SK, Sandhu A, Strommenger S. Clinical trials: a data driven feasibility approach: Pharmaceutical Outsourcing; 2017. https://www.pharmoutsourcing.com/Featured-Articles/333830-Clinical-Trials-A-Data-Driven-Feasibility-Approach/ . Accessed Jan 2019
Getz K. Is investigative site feasibility feasible? Appl Clin Trials. 2008; http://www.appliedclinicaltrialsonline.com/investigative-site-feasibility-feasible . Accessed Mar 2016.
Lamberti MJ, Chakravarthy R, Getz KA. Assessing practices and inefficiencies with site selection, study start-up, and site activation. Appl Clin Trials. 2016; http://www.appliedclinicaltrialsonline.com/assessing-practices-inefficiencies-site-selection-study-start-and-site-activation . Accessed Jan 2018.
Download references
Acknowledgements
The authors thank The Capital Region of Denmark and the Research Committee at Bispebjerg- and Frederiksberg hospital who funded this study.
This study was funded by a research grant from The Capital Region of Denmark and the Research Committee at Bispebjerg- and Frederiksberg hospital. The funders were not involved in the research, preparation, or writing of this article, nor in the decision to submit it for publication.
Author information
Authors and affiliations.
Department of Dermatology, Copenhagen University Hospital Bispebjerg, Bispebjerg Bakke 23, entrance 9, DK-2400, Copenhagen NV, Denmark
Tilde Dombernowsky, Merete Haedersdal & Simon Francis Thomsen
Department of Oncology, Copenhagen University Hospital Rigshospitalet, Blegdamsvej 9, entrance 5073, DK-2100, Copenhagen Ø, Denmark
Ulrik Lassen
Department of Biomedical Sciences, University of Copenhagen, Blegdamsvej 3b, DK-2200, Copenhagen N, Denmark
Simon Francis Thomsen
You can also search for this author in PubMed Google Scholar
Contributions
All authors were involved in the study design and contributed to the intellectual content of the manuscript. TD conducted the pretesting meetings and collected the data. Data analysis was conducted by TD and SFT. TD developed the first draft of the manuscript. MH, UL, and SFT contributed to the critical revision of the results and revised the manuscript. The final version of the manuscript is approved by all authors. TD is the gaurantor.
Corresponding author
Correspondence to Tilde Dombernowsky .
Ethics declarations
Ethics approval and consent to participate.
By Danish law, this study did not require ethics approval, approval from the Danish Data Protection Agency, or other regulatory approval. Informed consent was obtained from all participants. It was stressed that the results of the survey would be published without any disclosure of the respondents who chose to participate.
Consent for publication
Not applicable.
Competing interests
MH and SF declare relevant financial activities outside the submitted work: MH has received research grants from Leo Pharma, Lutronic, Novoxel, Procter and Gamble, and Sebacia, and declares loan of equipment from Cynosure Hologic, Lutronic, Novoxel, and Perfaction Technologies. SF has received research grants from AbbVie, Leo Pharma, Novartis, Sanofi, and UCB. SF has been a paid speaker for AbbVie, Eli Lilly, GSK, Leo Pharma, Novartis, Pierre Fabre, and Sanofi, and has served on advisory boards for Abbvie, Celgene, Eli Lilly, Janssen, Leo Pharma, Novartis, and Sanofi. UL has served on advisory boards for Bayer and Pfizer. TD declares to have no conflicts of interest.

Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: figure s1..
Information about trial sites that biopharmaceutical companies and CROs would find most valuable if available* * Respondents ( n = 83) were asked: Which information about a trial site that your company has not been cooperating with before would your company find the most valuable if available? The six response categories were ranked from one to six, one being the most valuable. CRO clinical research organizations, MR mean ranking (of the response category), SD standard deviation. Figure S2. Relative importance of site-related qualities while running early phase (A) and phase III trials (B)* * Respondents ( n = 83) were asked which of three site-related qualities the clinical operations departments at the affiliates of their company find the most important while running an early phase and phase III clinical trial, respectively. The three response categories were ranked from one to three, one being the most important. MR mean ranking (of the response category), SD standard deviation. Figure S3. The assessment of biopharmaceutical affiliates and CROs in early phase (A) and phase III trials (B)* * The biopharmaceutical-respondents ( n = 43) were asked which of four factors the headquarters of their company find the most important when evaluating the affiliates’ performance regarding running clinical trials. For CRO respondents ( n = 40), the question referred to the headquarters evaluation of the CRO. The four response categories were ranked from one to four, one being the most important. CRO clinical research organization, MR mean ranking (of the response category), SD standard deviation
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Dombernowsky, T., Haedersdal, M., Lassen, U. et al. Criteria for site selection in industry-sponsored clinical trials: a survey among decision-makers in biopharmaceutical companies and clinical research organizations. Trials 20 , 708 (2019). https://doi.org/10.1186/s13063-019-3790-9
Download citation
Received : 04 April 2019
Accepted : 05 October 2019
Published : 11 December 2019
DOI : https://doi.org/10.1186/s13063-019-3790-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Trial site selection
- Clinical trials
- Pharmaceutical industry
ISSN: 1745-6215
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Institute of Medicine (US) Committee for the Study of the Future of Public Health. The Future of Public Health. Washington (DC): National Academies Press (US); 1988.

The Future of Public Health.
- Hardcopy Version at National Academies Press
Appendix D Site Visits: Site Selection and Methodology
- Objectives for the Site Visits
Site visits to six states were included in the study plan in order to augment the information available to the committee about the current state of public health activities in the United States. The site visits were intended to elicit directly the perceptions of key actors who shape public health activities either through direct participation in those activities or through influence in the establishment of public health policies. The visits were conceived as an opportunity to learn more about the actual functioning of public health in addressing health problems—eliciting information that goes beyond formal organizational statements to probe more deeply into decision processes. This information would add to the committee's understanding of how policies are established and implemented and what problems are encountered that inhibit effective actions.
- Site Selection
The selection criteria aimed at a purposive sample that would maximize the opportunities for the committee to learn from visits to only six states—a constraint imposed by time and resources. A purposive sample was viewed by the committee as much more useful and valid than any attempt at randomization with such small numbers. The site visit information would augment other data available to the committee about all states (e.g., the data from the Public Health Foundation), statements at the four regional meetings, and the extensive knowledge and experience of the committee, including detailed knowledge among the committee of 13 additional states.
The selection process sought variety across the following dimensions:
- proportion of urban and rural population
- strength of economy, principal economic activities, and tax base
- intensity of public health activities (absolute and per capita expenditures)
- range of public health activities
- structure of government for public health (e.g., inclusion of public health in "superagency" versus independent public health agency)
- relative roles of state and local government in public health
- continuity of public health leadership
The intention was to visit localities within each state selected as well as the state capital. Therefore, additional opportunity was provided in the selection to represent diversity over these dimensions.
Data and other information on all the states were compiled by staff for consideration by the committee. In addition to the above dimensions, the intent was to choose states to the extent possible where the committee members were not currently active in administration of public health activities.
The final choices were made by the committee. The sites selected included the capitals and several local areas in each of the following states: West Virginia, New Jersey, California, Mississippi, South Dakota, and Washington.
- Methodology
The design of the site visits drew on the recent literature concerned with policy implementation research. (Pressman and Wildavskey, 1973; Nakamura and Smallwood, 1980; Sabatier and Nasmanian, 1980; Tooner, 1985; Williams, 1980) The emphasis of this research is on the transformation, within an administrative system, of inputs (laws, funds, personnel, techniques) into outputs (people served, regulatory activities performed, transfer payments made), and outcomes (lower infant mortality rate, control of infectious disease outbreaks, etc.). The effort is directed toward understanding what is actually going on at the day-to-day operational level of public problem-processing.
The site visits lasted 4 or 5 days. An advance visit was made by staff to arrange the visits. Committee and staff participated in each visit. Descriptive data on each state and locality were distributed to site visitors in advance. Interviewees were selected through interaction with informed persons in each state and locality visited. Interviewees included:
- public health officials (state and local and various levels within each organization)
- officials of related agencies (welfare, environment, Medicaid, etc.)
- elected officials (state and local)
- staff at general-purpose government levels (budget officials)
- practicing physicians and nurses
- other health leaders in the private sector (hospital associations, nursing associations, social worker associations)
- consumer leaders
- media representatives
The interview process was semistructured. An interview guide was provided to each interviewer outlining objectives, emphasizing the need to elicit the perceptions of those being interviewed without prejudgment from the interviewer, giving examples of interview techniques to help achieve this purpose and sample questions. The questions were aimed at eliciting not only description and evaluation but also hopes and aspirations for public health. Many of the questions were open-ended rather than highly structured in order to assure that the interviewees' perceptions were guiding the discussion.
A document describing the study and the purpose of the site visits was sent in advance to interviewees. That document outlined a series of nine specific health problems agreed to in advance by the committee in order to provide some initial focus for the interviews. Those problems were:
Tobacco Use and Addiction
Unintended Injuries
Hazardous Waste Disposal
Exposure to Asbestos
Pertussis Vaccine
Alzheimer's Disease
Medical Indigence
Teen-Age Pregnancy
The problem list was used as a starting point. The interviews did not attempt to confine the dimensions to those problems. (Indeed, some interviewees were quite forceful in adding other problems to the discussion.)
Each interviewer wrote up the interviews immediately following the interviews. A summary report on each visit was prepared by staff for the use of the full committee. As confidentiality was promised to interviewees, those reports will not be published. Information from all interviewees was classified by topic and entered into a computer system for later retrieval and analysis.
A shorter visit was made to the health department in Toronto, Canada. This 1-day visit did not follow the process described above, but was intended to provide some additional perspective on public health issues as seen from another political, social, and cultural context. Substantial information on public health activities in Toronto and Ontario was provided to the committee.
- Nakamura, Robert T., and F. Smallwood. 1980. The Politics of Policy Implementation . St. Martin's Press, New York.
- Pressman, Jeffrey L., and A. Wildavskey. 1973. Implementation . University of California Press, Berkeley.
- Sabatier, Paul, editor; , and R. Nasmanian, editor. , eds. 1980. Special Issue on Implementation . Policy Studies Journal , vol. 19.
- Tooner, Theo A. J. 1985. Implementation Research and Institutional Design: The Quest for Structure . Martinus Nijhoff, The Netherlands.
- Williams, Walter. 1980. The Implementation Perspective . University of California Press, Berkeley.
- Cite this Page Institute of Medicine (US) Committee for the Study of the Future of Public Health. The Future of Public Health. Washington (DC): National Academies Press (US); 1988. Appendix D, Site Visits: Site Selection and Methodology.
- PDF version of this title (2.2M)
In this Page
Recent activity.
- Site Visits: Site Selection and Methodology - The Future of Public Health Site Visits: Site Selection and Methodology - The Future of Public Health
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers
Advarra Clinical Research Network
Onsemble Community
Diversity, Equity, & Inclusion
Participants & Advocacy
Advarra Partner Network
Community Advocacy

Institutional Review Board (IRB)
Institutional Biosafety Committee (IBC)
Data Monitoring Committee (DMC)
Endpoint Adjudication (EAC)
GxP Services
Research Compliance & Site Operations
Professional Services
Coverage Analysis
Budget Negotiation
Calendar Build
Technology Staffing
Clinical Research Site Training
Research-Ready Training
Custom eLearning
Services for
Sponsors & CROs
Sites & Institutions
BioPharma & Medical Tech
Oncology Research
Decentralized Clinical Trials
Early Phase Research
Research Staffing
Cell and Gene Therapy
Ready to Increase Your Research Productivity?
Solutions for need.
Clinical Trial Management
Clinical Data Management
Research Administration
Study Startup
Site Support and Engagement
Secure Document Exchange
Research Site Cloud
Solutions for Sites
Enterprise Institution CTMS
Health System/Network/Site CTMS
Electronic Consenting System
eSource and Electronic Data Capture
eRegulatory Management System
Research ROI Reporting
Automated Participant Payments
Life Sciences Cloud
Solutions for Sponsors/CROs
Clinical Research Experience Technology
Center for IRB Intelligence
Insights for Feasibility
Not Sure Where To Start?
Resource library.
White Papers
Case Studies
Ask the Experts
Frequently Asked Questions
COVID-19 Support

About Advarra
Consulting Opportunities
Leadership Team
Our Experts
Accreditation & Compliance
Join Advarra
Learn more about our company team, careers, and values. Join Advarra’s Talented team to take on engaging work in a dynamic environment.
Strategies for Successful Site Selection in Clinical Trials
- Wendy Tate PhD, GStat Product Strategy Director;
Clinical trial site selection can make or break a trial’s success before it even begins. The average cost to open an investigator site is estimated at $50,000 1 – a price point compounding quickly when onboarding multiple sites. When you consider around 11% of sites 2 fail to even accrue one participant on a given study, cost savings become a major consideration when evaluating which sites to partner with for a trial.
To ensure you’re maximizing the time, resources, and funds associated for each clinical trial, we’ve outlined essential strategies for identifying the appropriate sites, analyzing historical site performance and feasibility, and implementing better communication and tools across stakeholders.
Analyzing Selection Criteria and Site Performance to Identify Your Sites
To identify clinical research sites for a study or trial opportunities, sponsors, contract research organizations (CROs), and sites leverage different datasets. Each sponsor collects data on the trials they’ve conducted and the performance of sites they’ve engaged in the past. A CRO may have more data across more therapeutic areas than a single sponsor. A site has historical performance data of their trials across a variety of sponsors and CROs, along with experience in specific clinical areas.
Each dataset is powerful because there is a lot to learn from past site and trial performance. In which trials have sites achieved their enrollment targets? What is a particular compliance rate with a specific site, or even a particular investigator across different studies? What selection criteria may be inhibiting your trial’s success? And do you have the resources to support sites with less experience in a particular clinical research area, but a strong relationship with a specific pool of patients?
Data like this can help solidify if a site will be a good fit for your unique trial and minimize the risk in your decision. However, each study stakeholder is limited in their decision-making by the dataset informing them. According to our recent trend report, The Current State of Trial Opportunity and Selection , 83% of the polled sites are looking for new trial opportunities, and over half of sponsor and CRO respondents indicated they utilize new-to-them research sites in at least half of their studies.
Consider using SiteIQ , a powerful tool designed to help sponsors and CROs simplify study startup and gain unique insight into site performance and identify high-performing sites currently not within their network. By leveraging Advarra’s data insights sourced from both sites and sponsors/CROs, SiteIQ highlights institution expertise, offers performance metrics from completed studies, and curates customized recommendations to support the needs of individual studies beyond your in-house dataset.
Factors Influencing Site Selection
Communication is an essential part of any effective clinical research relationship, and it’s important to always engage with each site thoroughly. Even if a particular clinical research site isn’t a fit for your current trial, they may be an important partner in future studies. Building these relationships will help you down the road, and can increase transparency between stakeholders.
There is a bi-directional need for communication. Sites need to feel comfortable being honest and transparent about how they think they’ll perform on a study. And even though they’re working with a lot of sites, sponsors need to allow some flexibility in getting information for the site to highlight why they’re an appropriate site for the study. This conversation gives sites the ability to communicate where their true strengths and weaknesses are.
An open line of communication can help to improve site management and engagement in the long term. The site evaluation process is a learning opportunity for everyone involved, and processes like study feasibility questionnaires (SFQs) can prove useful for all stakeholders involved. Our trend report found sponsor organizations are generally seeking to learn about four key evaluation items when selecting a site:
- Experience with patient population
- Clinical research experience
- Access to patient population
- Historical performance of enrollment
An SFQ introduces the opportunity for sites to answer sponsor’s key questions and provide additional information, but low site response rates have contributed to frustration—only about 65% of SFQs sponsors and CROs distribute are completed and returned. 3
On the other hand, sites are also frustrated by the lack of feedback. We found 83% of sites want more study opportunities, but rarely receive feedback from sponsors and CROs on why their site was not chosen. The lack of communication doesn’t help to foster site relationships, and missing insight likely contributes to low SFQ response rates.
Leveraging Technology in Site Selection
Technology is absolutely vital to a study, and you need a reliable way to store and collect data. Successful site and sponsor/CRO relationships are better enabled with tools designed to bring more insight to the conversation, while ensuring data is reproducible and properly communicated.
Oftentimes, relationships between sites and sponsors are very protocol-specific. Sponsors and CROs typically rely on an SFQ to gather site information. However, sites do not feel the SFQ allows them to properly highlight their abilities to conduct research. The sponsors rely on the SFQ and if the site doesn’t meet the criteria, they are not considered any longer. In some cases, this is in error as a follow-up conversation about the sponsor’s findings and probe of the site may find they actually are qualified and well suited to take on the study.
Communicating beyond these rigid questionnaires can allow for negotiation opportunities, potentially resulting in a highly successful site and clinical trial partner for a sponsor/CRO. The right tools can ensure mutual success, such as additional training resources, a financial feasibility analysis, and a centralized institutional review board (IRB) .
It’s important to remember there is a time frame between selecting and activating a site, during which these tools can be implemented. A typical academic medical center may take up to six months on average to activate a trial, while an independent site may take 30-45 days before enrolling patients. 4
Another essential step before activation is aligning on standard definitions for operational metrics — establishing common terminology when discussing how activation and trial conduct activities are proceeding. This step will optimize the workflow between all of the trial stakeholders, and can ensure the research is measured with the same set of conditions.
How Advarra can help
At Advarra, we work to advance clinical research through innovative solutions designed to leverage our industry expertise, optimizing your trial all the way from initial research to market.
To streamline site identification for your clinical trial, consider leveraging Advarra SiteIQ . The study planning tool was designed to simplify study startup by identifying suitable sites for your clinical trial using Advarra’s comprehensive database.
References:
- Optimizing Clinical Research Operations with Business Analytics, https://support.sas.com/resources/papers/proceedings11/204-2011.pdf
- Coalition for Clinical Trials Awareness, http://cctawareness.org/about-us/
- Trend Report: The Current State of Trial Opportunity and Selection
- JCO Oncology Practice, https://ascopubs.org/doi/full/10.1200/OP.19.00325
Tagged in: CRO , institutions , Site feasibility , site selection , Site selection metrics , sites , sponsors

Wendy expertise is focused on the quantitative evaluation of clinical research administration, bringing over 20 years of clinical research experience across various roles.
Back to Resources
To learn more about SiteIQ , schedule a demo today.
Subscribe to our monthly email.
Receive updates monthly about webinars for CEUs, white papers, podcasts, and more.
Advancing Clinical Research: Safer, Smarter, Faster ™
Copyright © 2024 Advarra. All Rights Reserved.
- Privacy Policy
- Terms of Use
- Cookie Policy

An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock A locked padlock ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.
https://www.nist.gov/news-events/news/2024/06/nine-finalists-2024-baldrige-award-get-site-visits
Nine Finalists for 2024 Baldrige Award to Get Site Visits
Nine U.S. organizations have been selected to receive site visits this summer, proceeding to the final phase of a significantly revised process for applicant assessments for the annual Baldrige Award . The award identifies national role models of excellence among U.S. businesses and other organizations that apply and receive rigorous evaluations of their performance against criteria that focus on resilience and long-term success.
Selection Process
During a virtual meeting last week, the 2024 Judges’ Panel reviewed ratings from the first phase of the award evaluation process, which extended from May to early June this year. To determine which organizations would receive site visits, the judges looked only at blinded data, thus avoiding any conflicts of interest and ensuring the integrity of the award process. The ratings were determined by evaluation teams composed of trained volunteers—members of the independent, all-volunteer Board of Examiners, who are competitively selected each year based on experience and expertise across various sectors of the U.S. economy.
Sixteen applicants were evaluated during the first phase of the 2024 Baldrige Award process. The nine organizations moving forward in the process will receive hybrid site visits this summer that will include virtual interviews of applicants, as well as two days of on-site examination by selected members of each evaluation team in late July.
The nine organizations that will receive site visits, of varying workforce sizes and sectors, include four health care organizations, three nonprofits (a category that includes government), one service business, and one education organization. These organizations may opt to receive public recognition as finalists for the 2024 Baldrige Award even if they are not selected as Baldrige Award recipients after the site visits.
Best Practice Spotlight
In addition, for the first time in 2024, some site-visited organizations not selected as Baldrige Award recipients may receive special recognition—a Best Practice Spotlight—for a process, activity, or initiative in an area of importance to the organization and/or the nation. The process, activity, or initiative would have a positive impact and strong results and be aligned with the 2024 Baldrige Award Criteria or the full 2023-2024 Baldrige Excellence Framework , including its 11 core values and concepts.
Streamlined Site Visit Process
During the upcoming site visits, examiner teams will verify organizational performance results in key areas, evaluate key processes that drove those results, and explore role-model characteristics. The approach has been streamlined to lower the burden on applicants and examiners. It begins with the applicant providing updated results and process information in advance of the site visit. The information provided will be analyzed by the examiner teams to determine what additional information is needed to complete their evaluations of the organizations. Engagement with the applicants will include virtual interviews as well as an in-person visit from two members of the examiner team.
Baldrige Judges' Panel
The Baldrige Award’s Judges’ Panel will reconvene in September to review findings about the site-visited organizations’ performance and determine which to recommend to the secretary of commerce to be named 2024 Baldrige Award recipients.
The Baldrige Performance Excellence Program (BPEP) is managed by the National Institute of Standards and Technology (NIST) in cooperation with the private sector and with support from the Foundation for the Malcolm Baldrige National Quality Award . BPEP raises awareness about the importance of performance excellence in driving the U.S. and global economies; provides organizational assessment tools and criteria; and educates leaders in all types of organizations. The Baldrige Award recognizes the nation’s top-level performers in six categories: manufacturing, service, small business, health care, education, and nonprofit. The Baldrige Award is not given for specific products or services. Since the first Baldrige Award recipients were recognized in 1988, 134 awards have been presented to 124 organizations (including nine two-time award recipients and one three-time award recipient).
- Skip to main content
- Skip to "About government"
Language selection
- Français

Prince Edward Island National Park
Gentle surf strokes sandy beaches alongside red cliffs and wind-sculpted dunes. Cycle a seashore path, savour a picnic by a lighthouse and spot heron wading in coastal bays. Hike woodlands and overlook ponds watching for red fox, waterfowl and warblers, then head to one of many beaches to build spectacular sandcastles. At sunset, roast marshmallows over a campfire listening to tales and songs - Prince Edward Island National Park is a giant playground for kids of all ages.
Make your Park Promise
The Park Promise is a commitment to taking better care of the earth, written by PEI poet laureate Julie Pellissier-Lush, a Mi'kmaq Knowledge Keeper from Lennox Island First Nation.
This initiative embodies our own hopes for a healthier, more sustainable future, and Parks Canada invites everyone to make the Promise and share their commitment to environmental stewardship. Learn more: Park Promise .

Camping in PEI National Park
Whether you prefer to camp in a tent, in a trailer, in accommodations or in the backcountry, we have the experience you're looking for!

Ultimate beach experience
Ready for a little fun in the sun? PEI National Park is famous around the world for its spectacular beaches, but that’s just the start.

Summer fun schedule
Discover fun things to do this summer within Prince Edward Island National Park.
Most requested
- Maps and brochures
- Passes and permits
- Supervised beaches
Plan your visit
- Dog walking
- Getting there
Visiting Prince Edward Island National Park
Maps and directions, fees, hours of operation, facilities and services.
Activities and experiences
Things to do, trails, interpretation programs, beaches and swimming, biking, and more.
Visitor Guidelines
Learn more about Prince Edward Island National Park guidelines and regulations currently in place.
Hours of operation
Find hours of operations for Prince Edward Island National Park.
Visitor Safety
Safety information, Prince Edward Island National Park of Canada.
Seasonal and annual admission passes.
About Prince Edward Island National Park
Culture and history.
History and culture in Prince Edward Island National Park.
Stewardship and management
Plans and policies, site management.
Nature and science
Nature and science in PEI National Park.
Important bulletins
Important bulletins and temporary road closures in PEI National Park.
Parks Canada administered land in French River - Park Corner
A large parcel of land around French River is now administered by Parks Canada.
_Location name
Telephone: 902-672-6350 Email: [email protected]
The park is accessible year-round. Visitor services are available from May to October.
More information
More places to discover with Parks Canada

Dalvay-by-the-Sea National Historic Site
Surrounded by sand dunes, beaches and a lake, Dalvay-by-the-Sea is a peek into a luxurious Victorian estate. Explore the interior, cycle the shoreline, stroll the grounds or simply relax in an Adirondack chair with a view.

Green Gables Heritage Place
Be charmed by an encounter with Canada’s iconic redheaded sweetheart, Anne of Green Gables. Relive the fictional orphan’s youthful adventures at the Island farmstead that inspired author Lucy Maud Montgomery.

Ardgowan National Historic Site
Stroll the serene and grand grounds surrounding Ardgowan, the former Charlottetown home of William Henry Pope, one of Canada’s Fathers of Confederation who resided and entertained in this cottage-style house during the Charlottetown Conference of 1864.

Province House National Historic Site
The birthplace of Confederation and the seat of Prince Edward Island's provincial legislature since 1847, Province House National Historic Site is a Charlottetown highlight. Stroll the grounds to experience the magnificent neo-classical architecture of this majestic building and view interpretive panels.

Skmaqn–Port-la-Joye–Fort Amherst National Historic Site
Established in 1720, Port-la-Joye was the entry point for European settlers coming to Île Saint-Jean to embark on a new life. There are centuries of history to discover in this historic location, declared a national historic site in 1967.
Language selection
- Français fr
Global Affairs Canada’s statement on visit to Xinjiang Autonomous Region by Canada’s Ambassador to China
From: Global Affairs Canada
Ambassador May’s visit to Xinjiang was part of Canada’s diplomatic engagement with Chinese officials and served as an opportunity to communicate Canadian concerns about the human rights situation directly to the leadership of Xinjiang.
June 23, 2024 - Ottawa, Ontario - Global Affairs Canada
Global Affairs Canada today issued the following statement on the visit of Ambassador of Canada to China Jennifer May to Xinjiang Uyghur Autonomous Region, China from June 19 to 22, 2024:
“Ambassador May’s visit to Xinjiang was part of Canada’s diplomatic engagement with Chinese officials and served as an opportunity to communicate Canadian concerns about the human rights situation directly to the leadership of Xinjiang.
“During her visit, Ambassador May met with Xinjiang Party Secretary MA Xingrui and other senior officials of the regional government of Xinjiang.
“Ambassador May raised concerns over credible reports of systematic violations of human rights occurring in Xinjiang affecting Uyghurs and other Muslim ethnic minorities, including those raised by UN experts, and the UN Office of the High Commissioner for Human Rights. She expressed Canada’s concerns over limits on Uyghur-language education and the practice of forcibly placing Uyghur children into residential schools.
“Ambassador May also reiterated Canada’s calls for China to allow UN independent experts unfettered access to all regions of China, including Xinjiang.
“Global Affairs Canada consulted key diaspora and civil society stakeholders in advance of this visit.”
Media Relations Office Global Affairs Canada [email protected] Follow us on Twitter: @CanadaFP Like us on Facebook: Canada’s foreign policy - Global Affairs Canada
Page details
Important Updates
Deserve for her own card
Programme of Study
- Bachelor of Design (B.Des.) at NID Ahmedabad
- Master of Design (M.Des.) at NID Ahmedabad, Gandhinagar & Bengaluru
- Bachelor of Design (B.Des.) at NID Andhra Pradesh
- Bachelor of Design (B.Des.) at NID Haryana
- Bachelor of Design (B.Des.) at NID Madhya Pradesh
- Bachelor of Design (B.Des.) at NID Assam
Fees Structure
- Bachelor of Design
- Master of Design
- Doctor of Philosophy (PhD) in Design
- ADMISSIONS HANDBOOK 2024-25
- Format (B.Des): Self Undertaking
- Format: SC/ST Certificate
- Format: OBC-NCL Certificate
- Format: EWS Certificate
- Format: PwD Certificate
- Format: Self Declaration by Scribe & Candidate
- Guidelines 29_08_2018
- Format (M.Des): Self-Undertaking by the candidate
- Format: Certificate issued by the University
- Format: Bonafide Certificate for B.Des.
- Format: Bonafide Certificate for M.Des.
- Format: Self Declaration regarding COVID-19
- Format: Self Declaration by Candidate & Scribe
- Format: Self Declaration by Candidate
- Format: Self Declaration by Scribe/Writer
- DAT Prelims Sample Test Papers
Announcements
- As on 27 March 2024, the revised date for the declaration of B.Des. DAT Prelims Result is Thursday, 04 April 2024
- As on 01 December 2023, an amendment has been made in the Admissions Handbook 2024-25 regarding extension of deadlines for submission of online applications and edit window.
US lawmakers meet Tibet's Dalai Lama, pressure China on talks
- Medium Text
- Influential US lawmakers hold talks with Dalai Lama in India
- Tibetans hail landmark act approved by Congress as breakthrough
- China opposes lawmakers’ meeting, Biden signing act
- Any change in US stance on Tibet will shock China, analysts say
TIBETANS HAIL BREAKTHROUGH

Sign up here.
Reporting by Charlotte Greenfield in Dharamsala, India, and David Brunnstrom in Washington; Additional reporting by Sunil Kataria; Writing by YP Rajesh; Editing by Clarence Fernandez and Sharon Singleton
Our Standards: The Thomson Reuters Trust Principles. New Tab , opens new tab

World Chevron

China's Chang'e-6 moon probe lands back on Earth
China's Chang'e-6 lunar probe landed on Tuesday in the northern Chinese region of Inner Mongolia, making the country the first to bring back samples from the moon's far side.

Trump says he knows who his vice presidential pick will be
PHILADELPHIA — Former President Donald Trump said Saturday that he knows who his vice presidential pick will be — and gave a clue about his or her likely debate night plans.
Asked by NBC News at a Philadelphia campaign stop whether he has decided on his vice presidential pick, Trump responded, “In my mind, yeah,” adding that the person will “most likely” be at Thursday’s debate against President Joe Biden.
“They’ll be there,” he continued. “I think we have a lot of people coming.”
Trump said “nobody knows” his choice yet. NBC News has previously reported that Trump is zeroing in on Gov. Doug Burgum of North Dakota, Sen. JD Vance of Ohio and Sen. Marco Rubio of Florida as possible top contenders.
Sources familiar with the selection process told NBC News that Burgum and Vance are considered the top two finalists. Rubio is still being considered, though a constitutional hiccup would require that either Trump or Rubio establish residency outside of Florida.
Trump senior adviser Brian Hughes said in a statement that the campaign’s top criterion for selecting a running mate “is a strong leader who will make a great President for eight years after his next four year term concludes.”
Trump is holding informal policy sessions with confidants in the lead-up to the debate, including possible vice presidential contenders. Vance spoke with Trump about the economy and inflation, and Rubio met with Trump when the former president was in Washington, D.C., this month.
His Saturday comments came during a stop at a Philadelphia restaurant ahead of a rally. Pennsylvania is expected to be a vital swing state on the road to the White House. Biden flipped Pennsylvania blue in 2020 and continues to campaign across the state as the election approaches.
The former president said he plans to announce his vice presidential pick “right around the convention.”
“Maybe a little before, but could be at the convention,” Trump said. “But we’ll have some great people.”
The Republican National Convention , at which delegates from across the country will officially nominate the party’s candidates for president and vice president, is set to be held July 15-18 in Milwaukee.
Jake Traylor and Dasha Burns reported from Philadelphia. Megan Lebowitz, Alec Hernández and Isabelle Schmeler reported from Washington, D.C.
Jake Traylor is a 2024 NBC News campaign embed.
Dasha Burns is a correspondent for NBC News.
Megan Lebowitz is a politics reporter for NBC News.
Alec Hernández is a 2024 NBC News campaign embed.
Isabelle Schmeler is a researcher on the 2024 politics desk.
Once You Finish the NYT's Games, Visit This Puzzle Site Next

Your changes have been saved
Email Is sent
Please verify your email address.
You’ve reached your account maximum for followed topics.
Key Takeaways
- Puzzmo offers free daily puzzles, including unique games like Cross|Word and Flipart.
- The Pile-Up Poker game adds a poker twist to Sudoku concept for a fresh challenge.
- While Puzzmo is free, there is a subscription available to remove ads.
You may have become addicted to the New York Times’ suite of games—titles like Wordle, Connections, and Spelling Bee. But if you’re anything like me, you find they're over too quickly and wish you could play more. Fortunately, Puzzmo is here to scratch that itch.
What Is Puzzmo?
Puzzmo is a gaming platform offering a suite of short puzzle games that can all be played in a few minutes. It’s the co-creation of indie game developer Zach Gage, who you may know from iOS titles like SpellTower and TypeShift .
The site is owned by Hearst, which means you’re likely to see Puzzmo puzzles pop up in places like the San Francisco Chronicle. But you can play most of the games on Puzzmo.com for free; each game refreshes daily with a new puzzle to solve, so you don’t have to worry about any of them going stale.
It's the perfect site for those that enjoy quick web games .
What Kind of Games Does Puzzmo Have?
If you like word or spelling games , you’re in luck, because those comprise the bulk of the offerings on Puzzmo. But even fans of graphical puzzles or logic games will find something to like here, and the site is constantly experimenting with new titles.
Here are some of the best...
Cross|Word is, as the title says, a crossword puzzle. Read the clues and try to fill in each square. If you get stuck you can click on the Hint button for another clue.
What makes Cross|Word stand out from other crosswords on the web (including the NYT) is the focus of its word selection: instead of being packed with literary and historical references, Puzzmo’s take on the format has a more Millennial/Gen Z bent, with plenty of clues based on internet slang, video games, and even anime.
But more traditional players need not fret, as there are still plenty of clues dabbling in historical figures, geography, and the Greek alphabet.
Pile-Up Poker
Pile-Up Poker is a newer Puzzmo game. It’s basically a poker-themed Sudoku, presenting players with a 4x4 grid and cards that must be placed on the grid to create poker hands.
Each game of Pile-Up Poker is played over four rounds, and you’re given five cards at a time. You can place four of them on the board anywhere you want, and the fifth card is discarded. Once the board is filled, a score is calculated based on created hands (both horizontally and vertically)—even if the discarded cards form a hand on their own!
In Flipart , you must fit a series of different pieces neatly inside a box, with no overlaps or empty spaces. The pieces are pretty diverse, ranging from squares to various Z-shapes to long straight pieces; a bit like Tetris on steroids.
The pieces are stuck in place at a single point, so you can’t drag them to where they need to go, only rotate them around a single square (helpfully indicated with a series of dots). Once you’ve mastered the rules and understand how different pieces work together, you’ll be solving each day’s puzzle in no time. My personal best is 12 seconds; can you beat it?
Really Bad Chess
Don’t let the name fool you— Really Bad Chess is an excellent game. The “really bad” moniker comes more from how it’s likely to annoy many chess purists. At first, it seems like a normal chess game, where you must checkmate your computer opponent.
But look at the pieces, and you’ll see that you don’t have the standard selection of king, queen, two bishops, two knights, two rooks, and eight pawns. Instead, the assortment is almost completely random, and so is your opponent's.
While each side will always have one king, you could end up with three queens, five bishops, and no pawns at all! Whatever you end up with, the goal is still to go after the other side’s king—and, if your computer opponent has more powerful pieces than you, this can be rather tricky.
How Much Does Puzzmo Cost?
Just like the basic NYT games suite, Puzzmo is free—as long as you’re willing to watch some ads. The frequency of ads isn’t annoying; you’ll get maybe one per day, right before starting one of the games. It’s just a short ad followed by a Puzzmo promo roll, then you’ll get to enjoy your puzzle-y goodness.
However, if you want more than just the small selection of daily puzzles, then you’ll have to subscribe to Puzzmo Plus which, like NYT Games, grants access to the entire archive. It also grants access to experimental games: ones that aren’t quite ready for the big time but can get there thanks to playtesting and feedback from users like you.
Puzzmo Plus also offers leaderboards and customizable avatars, if that’s your thing. All of these features are available for $39 a year (no monthly option is available). That’s $10 less than a yearly subscription to NYT Games , which is $50 a year, though that service does periodically offer discounts that make it cheaper with more features (like access to other New York Times content).
If you’re curious about Puzzmo Plus but not ready to hand over $39 just yet, the site offers a free two-week trial.
What Are Puzzmo's Best Features and Why Should You Use It?
As I’ve already outlined, the quality of games at Puzzmo is extremely high, thanks to the titles I mentioned above and others like SpellTower and Typeshift, the latter of which is part of my daily rotation.
Even though the site only launched in 2023, its archive is already pretty extensive, making a Puzzmo Plus account well worth its cost for those days you need some serious mental stimulation. And competitive users will really appreciate the sheer amount of score and time info the site keeps.
But, even if you play for free and don’t care about stats, Puzzmo is still worth a daily visit during your lunch break or before bedtime, especially once you’ve played out all the New York Times’ options.
- Browser Games
- Puzzle Games

IMAGES
VIDEO
COMMENTS
Effective Date: 01-JUL-2017 Site Qualification Visit Page 1 of 4 . SOP-05: Site Qualification Visit . 1. Objective To ensure that the Principal Investigator (PI) and all research team members assisting in the conduct of clinical research are informed about their obligations and responsibilities as they pertain to Good Clinical Practices
Learn how a Clinical Research Associate (CRA) prepares and conducts a Site Initiation Visit (SIV) to activate a clinical trial site. The article covers the objectives, steps, and responsibilities of a SIV, as well as the documents and items needed for the visit.
Learn about the types and purposes of site monitor visits for clinical research studies, including pre-study visits (site selection visits or site qualification visits). Find out how to prepare for and host site monitor visits at OSU.
Learn how to choose clinical sites that can meet enrollment goals, ethical standards, and regulatory requirements. This article covers practical, protocol, and ethical considerations, as well as facilities, recruitment, and retention strategies.
This document outlines the procedures for conducting a pre-study site visit at Beaumont Health, a health system in Michigan, USA. It covers the steps from scheduling, preparing, and following up the visit with a research sponsor or CRO.
Learn how clinical research sites are awarded studies through feasibility surveys and site selection visits (SSV) by sponsors. Find out what CRA's look for and observe during SSV and how sites can prepare and impress them.
Initial (first)monitoring visit. If you were recently hired for a CRA position in a new pharmaceutical company, you would need to do the next steps prior to scheduling the first monitoring visit: - Familiarize with the company's general SOPs and Sponsor's study-specific SOPs (if applicable) relating to the clinical study initiation ...
Site Selection. Your participation in choosing the site(s) for the clinical study can help mitigate patient risk and attract participants, as well as providing useful knowledge of day-to-day living and general practicalities to researchers. Geographic and travel information is vital to the site selection process. Researchers may also be unaware ...
This chapter describes the criteria for selecting clinical trial sites, how to set up a site and prepare for site selection visits. It also describes the qualities a site should have and the necessity of adequate, qualified staff for the duration of the trial. Sponsors would normally appoint a Clinical Research Organisation (CRO) to conduct an ...
This will increase the likelihood of a trial that is both timely and cost-effective. Today, we are giving investors and clinical research recruiters a brief guide on what to look for in research sites; and what research sites should be doing to make sure they impress. Check out our 2020 guide below.
All confirmation, follow-up letters and Site Initiation Visit Report (per GCP requirements) are to be properly stored in Investigator Site File located at Sites. Pre-Study Site Selection Visit (PSSV) This is the starting point of interactions between Sponsor/CRO and Clinical Trial Site.
Here are some best practices for conducting a successful site initiation visit: Schedule the site initiation visit as early as possible in the study start-up process to allow sufficient time for addressing any issues that may arise. Confirm that the site has all the necessary study documents, including the protocol, informed consent form, case ...
Join this channel to get access to perks:https://www.youtube.com/channel/UCvw9kVKHEyAlZPZ6ZuOd2VA/joinText Me: (949) 415-6256My podcast is Random Musings Fro...
Site Qualification visits are an essential component of the clinical trials site selection process. This visit allows both you and the trial's Sponsor to learn more about each other and ascertain if the study is a good fit. It is also your chance to show that you and your facility can support the many regulatory and performance requirements that each clinical study demands.
SIV Definition: Site initiation visit. An SIV (clinical trial site initiation visit) is a preliminary inspection of the trial site by the sponsor before the enrollment and screening process begins at that site. It is generally conducted by a monitor or clinical research associate (CRA), who reviews all aspects of the trial with the site staff ...
No site selection visit was performed to verify the suitability of sites to be part of the GFC study. Unfortunately, after the contracting process was started, two sites in one country had to be replaced. One site declined due to the workload caused by its participation in the study, which was underestimated by the site itself. ...
#SiteSelectionVisit #PreStudyVisit #ClinicalResearchAssociate #CRAI offer resume review review, interview prep, mock interviews and mentoring to those intere...
In summary, a site selection visit that ensures facilities and qualified staff are in place is a relatively straightforward process. However, determining if a site will actually recruit patients into the trial is another matter entirely. In order to make the process more effective and successful, we need to start by creating a culture and ...
This article presents standard operating procedures (SOPs) for the selection and assessment of study sites and investigators for clinical research. It includes SOPs for initial selection process, site selection visits, assessment of Phase I facilities, and documentation of qualifications of site personnel.
Knowledge of what the pharmaceutical industry emphasizes when assessing trial sites during site selection is sparse. A better understanding of this issue can improve the collaboration on clinical trials and increase knowledge of how to attract and retain industry-sponsored trials. Accordingly, we investigated which site-related qualities multinational biopharmaceutical companies and clinical ...
The process of site selection is both an art and a science. The better the quality of information gathered, the better the decision making. The more you can validate about the sites' access to the appropriate patient population, the higher the likelihood of enrollment success.9,10 Effective site selection in combination with a robust recruitment
Site Selection. The selection criteria aimed at a purposive sample that would maximize the opportunities for the committee to learn from visits to only six states—a constraint imposed by time and resources. ... This 1-day visit did not follow the process described above, but was intended to provide some additional perspective on public health ...
Clinical trial site selection can make or break a trial's success before it even begins. The average cost to open an investigator site is estimated at $50,000 1 - a price point compounding quickly when onboarding multiple sites. When you consider around 11% of sites 2 fail to even accrue one participant on a given study, cost savings become a major consideration when evaluating which sites ...
Selection Process. During a virtual meeting last week, the 2024 Judges' Panel reviewed ratings from the first phase of the award evaluation process, which extended from May to early June this year. To determine which organizations would receive site visits, the judges looked only at blinded data, thus avoiding any conflicts of interest and ...
The birthplace of Confederation and the seat of Prince Edward Island's provincial legislature since 1847, Province House National Historic Site is a Charlottetown highlight. Stroll the grounds to experience the magnificent neo-classical architecture of this majestic building and view interpretive panels.
Ambassador May's visit to Xinjiang was part of Canada's diplomatic engagement with Chinese officials and served as an opportunity to communicate Canadian concerns about the human rights situation directly to the leadership of Xinjiang. June 23, 2024 - Ottawa, Ontario - Global Affairs Canada ...
As on 27 March 2024, the revised date for the declaration of B.Des. DAT Prelims Result is Thursday, 04 April 2024; As on 08 February 2024 the tie-break policy for B.Des. & M.Des. DAT Prelims and Final result has been announced in the Amendments Section of the Admissions Handbook 2024-25.
The U.S. group, which includes Democratic former House Speaker Nancy Pelosi, arrived on Tuesday for a two-day visit. Item 1 of 2 Former U.S. House speaker Nancy Pelosi speaks at the Tibetan ...
Former President Donald Trump said in Philadelphia that he knows who his vice presidential pick will be — and gave a clue about his or her likely debate night plans.
Cross|Word is, as the title says, a crossword puzzle. Read the clues and try to fill in each square. If you get stuck you can click on the Hint button for another clue.. What makes Cross|Word stand out from other crosswords on the web (including the NYT) is the focus of its word selection: instead of being packed with literary and historical references, Puzzmo's take on the format has a more ...