- Bipolar Disorder
- Therapy Center
- When To See a Therapist
- Types of Therapy
- Best Online Therapy
- Best Couples Therapy
- Managing Stress
- Sleep and Dreaming
- Understanding Emotions
- Self-Improvement
- Healthy Relationships
- Student Resources
- Personality Types
- Sweepstakes
- Guided Meditations
- Verywell Mind Insights
- 2024 Verywell Mind 25
- Mental Health in the Classroom
- Editorial Process
- Meet Our Review Board
- Crisis Support

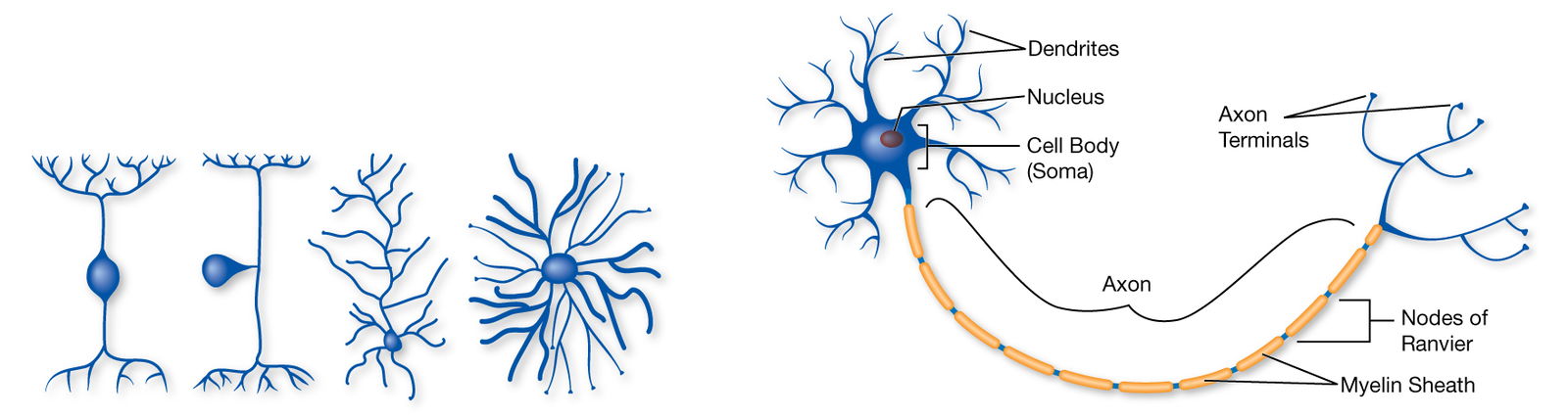
An Overview of the Different Parts of a Neuron
From Dendrites to the Terminal Buttons Found at the End of Axons
Neurons are the basic building blocks of the nervous system. These specialized cells are the information-processing units of the brain responsible for receiving and transmitting information. Each part of the neuron, from the dendrite to the terminal buttons found at the end of the axon, plays a role in communicating information throughout the body.
Neurons carry messages throughout the body, including sensory information from external stimuli and signals from the brain to different muscle groups in the body. In order to understand exactly how a neuron works, it is important to look at each individual part of the neuron. The unique structures of the neuron allow it to receive and transmit signals to other neurons as well as other types of cells.
Dendrites are tree-like extensions at the beginning of a neuron that help increase the surface area of the cell body. These tiny protrusions receive information from other neurons and transmit electrical stimulation to the soma. Dendrites are also covered with synapses.
Characteristics
- Have many dendrites, or only one dendrite
- Are short and highly branched
- Transmit information to the cell body
Most neurons possess these branch-like extensions that extend outward away from the cell body. These dendrites then receive chemical signals from other neurons, which are then converted into electrical impulses that are transmitted toward the cell body.
Some neurons have very small, short dendrites, while other cells possess very long ones. The neurons of the central nervous systems have very long and complex dendrites that then receive signals from as many as a thousand other neurons.
If the electrical impulses transmitted inward toward the cell body are large enough, they will generate an action potential. This results in the signal being transmitted down the axon.
The soma, or cell body, is where the signals from the dendrites are joined and passed on. The soma and the nucleus do not play an active role in the transmission of the neural signal. Instead, these two structures serve to maintain the cell and keep the neuron functional.
- Contains numerous organelles involved in a variety of cell functions
- Contains a cell nucleus that produces RNA that directs the synthesis of proteins
- Supports and maintains the functioning of the neuron
Think of the cell body as a small factory that fuels the neuron.
The soma produces the proteins that the other parts of the neuron, including the dendrites, axons, and synapses, need to function properly.
The support structures of the cell include mitochondria, which provide energy for the cell, and the Golgi apparatus, which packages products created by the cell and dispatches them to various locations inside and outside the cell.
Axon Hillock
The axon hillock is located at the end of the soma and controls the firing of the neuron. If the total strength of the signal exceeds the threshold limit of the axon hillock, the structure will fire a signal (known as an action potential ) down the axon.
The axon hillock acts as something of a manager, summing the total inhibitory and excitatory signals. If the sum of these signals exceeds a certain threshold, the action potential will be triggered and an electrical signal will then be transmitted down the axon away from the cell body. This action potential is caused by changes in ion channels which are affected by changes in polarization.
- Acts as something of a manager, summing the total inhibitory
- Possesses an internal polarization of approximately -70mV in a normal resting state
When a signal is received by the cell, it causes sodium ions to enter the cell and reduce polarization. If the axon hillock is depolarized to a certain threshold, an action potential will fire and transmit the electrical signal down the axon to the synapses.
It is important to note that the action potential is an all-or-nothing process and that signals are not partially transmitted. The neurons either fire or they do not.
The axon is the elongated fiber that extends from the cell body to the terminal endings and transmits the neural signal. The larger the diameter of the axon, the faster it transmits information.
Some axons are covered with a fatty substance called myelin that acts as an insulator. These myelinated axons transmit information much faster than other neurons.
- Most neurons have only one axon
- Transmit information away from the cell body
- May or may not have a myelin covering
- Range dramatically in size, from 0.1 millimeters to over 3 feet long
The myelin surrounding the neurons protects the axon and aids in the speed of transmission. The myelin sheath is broken up by points known as the nodes of Ranvier or myelin sheath gaps. Electrical impulses are able to jump from one node to the next, which plays a role in speeding up the transmission of the signal.
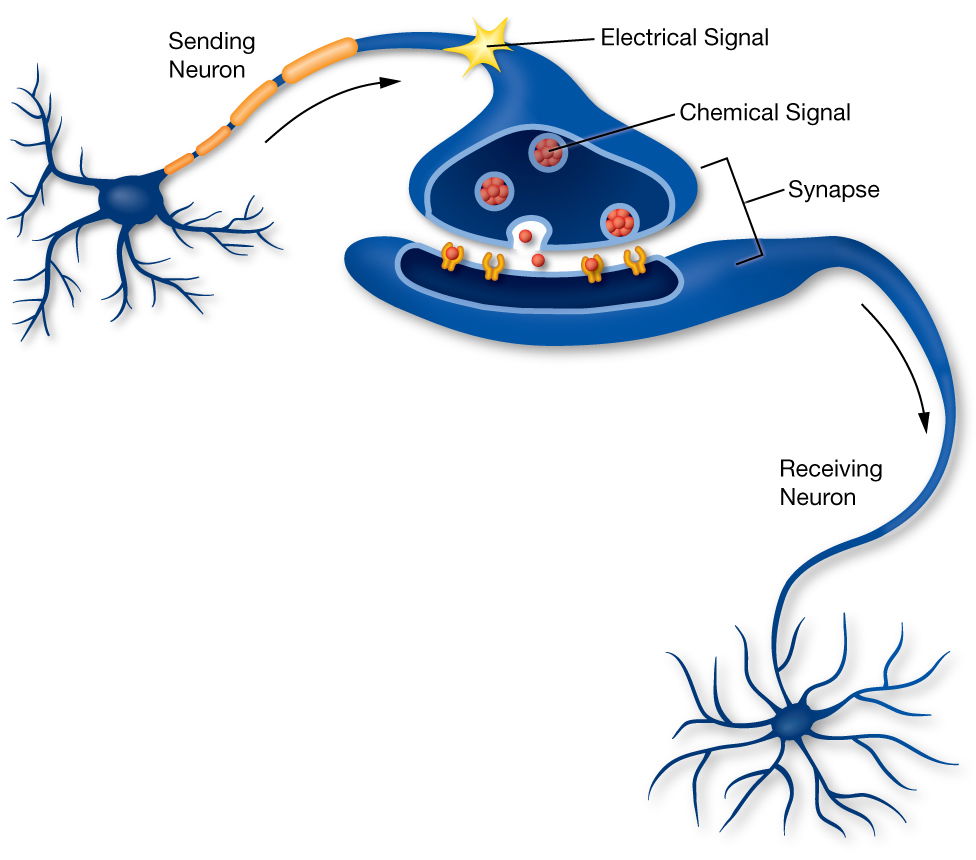
Axons connect with other cells in the body including other neurons, muscle cells, and organs. These connections occur at junctions known as synapses.
The synapses allow electrical and chemical messages to be transmitted from the neuron to the other cells in the body.
Terminal Buttons and Synapses
Terminal buttons are found at the end of the axon, below the myelin sheath, and are responsible for sending the signal on to other neurons. At the end of the terminal button is a gap known as a synapse.
Neurotransmitters carry signals across the synapse to other neurons. When an electrical signal reaches the terminal buttons, neurotransmitters are then released into the synaptic gap.
- Contain vesicles holding the neurotransmitters
- Convert electrical impulses into chemical signals
- Cross the synapse where they are received by other nerve cells
- Responsible for the reuptake of any excessive neurotransmitters released during this process
A Word From Verywell
Neurons serve as basic building blocks of the nervous system and are responsible for communicating messages throughout the body.
Knowing more about the different parts of the neuron can help you to better understand how these important structures function as well as how different problems, such as diseases that impact axon myelination, might impact how messages are communicated throughout the body.
Luengo-Sanchez S, Bielza C, Benavides-Piccione R, Fernaud-Espinosa I, DeFelipe J, Larrañaga P. A univocal definition of the neuronal soma morphology using Gaussian mixture models . Front Neuroanat . 2015;9:137. doi:10.3389/fnana.2015.00137
Miller AD, Zachary JF. Nervous System . In: Zachary JF, ed. Pathologic Basis of Veterinary Disease . St. Louis, MO: Mosby, Inc.; 2017. doi:10.1016/B978-0-323-35775-3.00014-X
Debanne D, Campana E, Bialowas A, Carlier E, Alcaraz G. Axon Physiology . Psychol Rev. 2011;91(2):555-602 . doi:10.1152/physrev.00048.2009
By Kendra Cherry, MSEd Kendra Cherry, MS, is a psychosocial rehabilitation specialist, psychology educator, and author of the "Everything Psychology Book."
- Virtual Tour
- Ask the Brain
- Message from the Director
- The McGoverns
- Administration
- Explore the Brain
- Polina Anikeeva
- Emilio Bizzi
- Martha Constantine-Paton
- Robert Desimone
- James DiCarlo
- Evelina Fedorenko
- Michale Fee
- Guoping Feng
- John Gabrieli
- Ann Graybiel
- Mark Harnett
- H. Robert Horvitz
- Alan Jasanoff
- Mehrdad Jazayeri
- Nancy Kanwisher
- Josh McDermott
- Tomaso Poggio
- Rebecca Saxe
- Nidhi Seethapathi
- Satrajit Ghosh
- Dimitrios Pantazis
- Ian Wickersham
- Brain Imaging
- Cellular & Molecular Neuroscience
- Cognitive Neuroscience
- Computational Neuroscience
- Genome Engineering
- Neurotechnology
- Systems Neuroscience
- Alzheimer’s Disease
- Autism Spectrum Disorder
- Bipolar Disorder
- Huntington’s Disease
- Obsessive-Compulsive Disorder
- Parkinson’s Disease
- Schizophrenia
- Participate in a Study
- Community Resources
- Athinoula A. Martinos Imaging Center
- Poitras Center for Psychiatric Disorders Research
- Yang Tan Collective
- Join Our Mailing List
- Newsletter Archive
- Sponsored Researchers
- Meet Our Supporters
- Events Calendar
- McGovern Institute Annual Symposium
- Edward M. Scolnick Prize in Neuroscience
- Phillip A. Sharp Lecture in Neural Circuits
We take a closer look at the anatomy of the neuron and the role myelin plays in the rapid transmission of messages between brain cells.
In the neuron, a protective covering called myelin (grey) insulates the axon and increases the speed of electrical communication along the length of the neuron. Image: Opus Design
How do neurons communicate (so quickly)?
by Sabbi Lall | February 28, 2019 May 24, 2023
Categories: Cellular & Molecular Neuroscience , Guoping Feng , Ask the Brain
Neurons are the most fundamental unit of the nervous system, and yet, researchers are just beginning to understand how they perform the complex computations that underlie our behavior. We asked Boaz Barak , previously a postdoc in Guoping Feng ’s lab at the McGovern Institute and now Senior Lecturer at the School of Psychological Sciences and Sagol School of Neuroscience at Tel Aviv University, to unpack the basics of neuron communication for us.
“Neurons communicate with each other through electrical and chemical signals,” explains Barak. “The electrical signal, or action potential, runs from the cell body area to the axon terminals, through a thin fiber called axon. Some of these axons can be very long and most of them are very short. The electrical signal that runs along the axon is based on ion movement. The speed of the signal transmission is influenced by an insulating layer called myelin,” he explains.
Myelin is a fatty layer formed, in the vertebrate central nervous system, by concentric wrapping of oligodendrocyte cell processes around axons. The term “myelin” was coined in 1854 by Virchow (whose penchant for Greek and for naming new structures also led to the terms amyloid, leukemia, and chromatin). In more modern images, the myelin sheath is beautifully visible as concentric spirals surrounding the “tube” of the axon itself. Neurons in the peripheral nervous system are also myelinated, but the cells responsible for myelination are Schwann cells, rather than oligodendrocytes.
“Neurons communicate with each other through electrical and chemical signals,” explains Boaz Barak.
“Myelin’s main purpose is to insulate the neuron’s axon,” Barak says. “It speeds up conductivity and the transmission of electrical impulses. Myelin promotes fast transmission of electrical signals mainly by affecting two factors: 1) increasing electrical resistance, or reducing leakage of the electrical signal and ions along the axon, “trapping” them inside the axon and 2) decreasing membrane capacitance by increasing the distance between conducting materials inside the axon (intracellular fluids) and outside of it (extracellular fluids).”
Adjacent sections of axon in a given neuron are each surrounded by a distinct myelin sheath. Unmyelinated gaps between adjacent ensheathed regions of the axon are called Nodes of Ranvier, and are critical to fast transmission of action potentials, in what is termed “saltatory conduction.” A useful analogy is that if the axon itself is like an electrical wire, myelin is like insulation that surrounds it, speeding up impulse propagation, and overcoming the decrease in action potential size that would occur during transmission along a naked axon due to electrical signal leakage, how the myelin sheath promotes fast transmission that allows neurons to transmit information long distances in a timely fashion in the vertebrate nervous system.
Myelin seems to be critical to healthy functioning of the nervous system; in fact, disruptions in the myelin sheath have been linked to a variety of disorders.

“Abnormal myelination can arise from abnormal development caused by genetic alterations,” Barak explains further. “Demyelination can even occur, due to an autoimmune response, trauma, and other causes. In neurological conditions in which myelin properties are abnormal, as in the case of lesions or plaques, signal transmission can be affected. For example, defects in myelin can lead to lack of neuronal communication, as there may be a delay or reduction in transmission of electrical and chemical signals. Also, in cases of abnormal myelination, it is possible that the synchronicity of brain region activity might be affected, for example, leading to improper actions and behaviors.”
Researchers are still working to fully understand the role of myelin in disorders. Myelin has a long history of being evasive though, with its origins in the central nervous system being unclear for many years. For a period of time, the origin of myelin was thought to be the axon itself, and it was only after initial discovery (by Robertson, 1899), re-discovery (Del Rio-Hortega, 1919), and skepticism followed by eventual confirmation, that the role of oligodendrocytes in forming myelin became clear. With modern imaging and genetic tools, we should be able to increasingly understand its role in the healthy, as well as a compromised, nervous system.
Do you have a question for The Brain? Ask it here .
Finding some stability in adaptable brains

Harnessing the power of placebo for pain relief
Scientists find neurons that process language on different timescales
- Basic Neuroscience
Neurons Transmit Messages In The Brain
Neurons Communicate via the Synapse
Information from one neuron flows to another neuron across a small gap called a synapse (SIN-aps). At the synapse, electrical signals are translated into chemical signals in order to cross the gap. Once on the other side, the signal becomes electrical again.
One sending neuron can connect to several receiving neurons, and one receiving neuron can connect to several sending neurons.

12.4 Communication Between Neurons
Learning objectives.
By the end of this section, you will be able to:
Describe signal conduction at chemical synapses.
- Describe the steps of the chemical synapse
- Explain the differences between the types of graded potentials, including ions involved
- Categorize the major neurotransmitters by chemical type and effect
A synapse is the site of communication between a neuron and another cell. There are two types of synapses: chemical synapses and electrical synapses . In a chemical synapse, a chemical signal— a neurotransmitter—is released from the neuron and it binds to a receptor on the other cell. In an electrical synapse, the membranes of two cells directly connect through a gap junction so that ions can pass directly from one cell to the next, transmitting a signal. Both types of synapses occur in the nervous system, though chemical synapses are more common.
An example of a chemical synapse is the neuromuscular junction (NMJ) described in the chapter on muscle tissue. In the nervous system, there are many additional synapses that utilize the same mechanisms as the NMJ. All chemical synapses have common characteristics, which can be summarized in Table 12.2 :
Neurotransmitter Release
When an action potential reaches the axon terminals, voltage-gated Ca 2+ channels in the membrane of the synaptic end bulb open. Ca 2+ diffuses down its concentration gradient and enters into the presynaptic neuron axon terminal (end bulb). Once Ca 2+ is inside the presynaptic end bulb, it associates with proteins to trigger the exocytosis of neurotransmitter vesicles. The released neurotransmitter moves into the small gap between the cells, the synaptic cleft .
Once in the synaptic cleft, the neurotransmitter diffuses the short distance to the postsynaptic membrane and can bind to neurotransmitter receptors. Receptors are specific for the neurotransmitter, and the two fit together like a lock and key, and so a neurotransmitter will not bind to receptors for other neurotransmitters ( Figure 12.4.1 ).
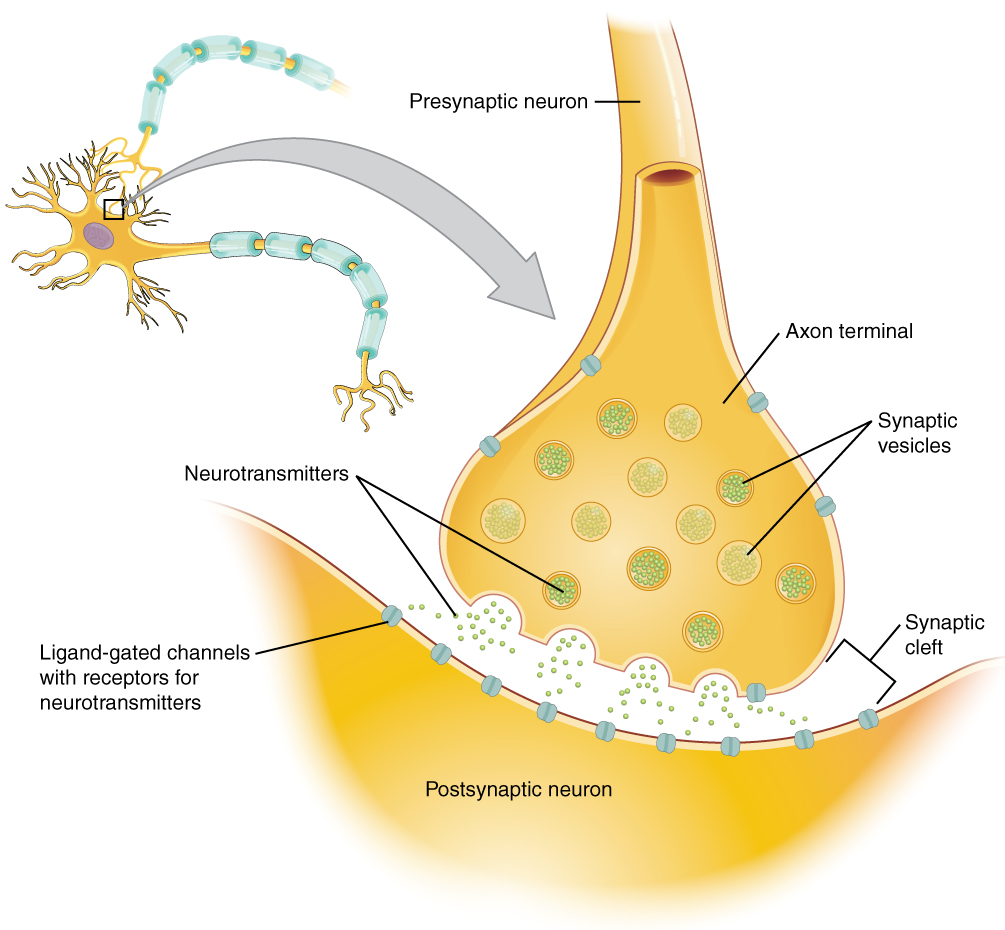
Neurotransmitter and Receptor Systems
Neurotransmitters vary greatly throughout the body, but one principle applies to all: neurotransmitters must bind to their own specific receptor, of which there can be subtypes. We will use acetylcholine (neurotransmitter) and its receptor (cholinergic) as an example. There are two subtypes of cholinergic receptors both of which bind acetylecholine: nicotinic receptors and muscarinic receptors (their names are based on the other chemicals that can also bind to the receptor). Nicotine will bind to the nicotinic receptor and activate it, just like acetylcholine. Muscarine, a product of certain mushrooms, will bind to the muscarinic receptor, just like acetylcholine. However, nicotine will not bind to the muscarinic receptor and muscarine will not bind to the nicotinic receptor. Skeletal muscle NMJs always involve nicotinic cholinergic receptors and when acteylcholine binds to nicotinic receptors, a Na + ligand gated channel opens. Muscarinic receptors are found sometimes with with K + ligand gated channels and other times with Na + ligand gated channels, differing throughout the body. For example, when acetylcholine binds to a muscarinic receptor on the pace-maker cells of the heart, K + ligand gated channels open and heart rate slows down. When acetylcholine binds to a muscarinic receptor on the small intestine muscle, a Na + ligand gated channel opens and the muscle activates (contracts). This variability in receptor/channel combinations is common throughout the body and occurs for many other neurotransmitters like epinephrine (adrenaline), serotonin and dopamine.
Neurotransmitters are classified in many ways based on their structural chemical make up or their functional common effects. Chemically, neurotransmitters can be small, amino acid based molecules, released from neurons as amino acids themselves (ie: glutamate, glycine) or as enzymatically modified relatively simple molecules (acetylecholine, ATP or biogenic amines such as dopamine). Larger molecule neurotransmitters are more complex proteins (3-36 amino acids long) called neuropeptides. There are more than 100 different peptides and include those such as enkephalins or endorphins, each with their own receptor types and subtypes that bind them.
Types of Neurotransmitters
Small molecule neurotransmitters: amino acids, acetylcholine, and purine neurotransmitters.
Amino Acids : Glutamate (Glu), GABA (gamma-aminobutyric acid, a derivative of glutamate), and glycine (Gly) are common amino acid neurotransmitters. These amino acids have an amino group and a carboxyl group in their chemical structures. Glutamate is one of the 20 amino acids that are used to make proteins. Each amino acid neurotransmitter would be part of its own system, namely the glutamatergic, GABAergic, and glycinergic systems. They each have their own receptors and do not interact with each other. Amino acid neurotransmitters are eliminated from the synapse by reuptake in the neuron that released them. A pump in the presynaptic cell membrane, or sometimes a neighboring glial cell, removes the amino acid from the synaptic cleft so that it can be recycled, repackaged in vesicles, and released again.
The amino acid neurotransmitters, glutamate, glycine, and GABA, are almost exclusively associated with just one effect. Glutamate is often considered an excitatory amino acid, but only because glutamate receptors in the adult cause depolarization of the postsynaptic cell (by changing membrane permeability to Na + or Ca 2+ ). Glycine and GABA are considered inhibitory amino acids, because their receptors typically cause hyperpolarization (by chaniging membrane permability to Cl – or K + ).
Acetylcholine and ATP : Acetylcholine was described above, including its excitatory or inhibitor effects when binding to various cholinergic receptors. ATP, the energy molecule and a purine chemically, has been found to act as a neurotransmitter in both the peripheral and central nervous system, often associated with excitatory effects.
Small Molecule Neurotransmitters: Biogenic Amines
Biogenic amines are a group of neurotransmitters that are enzymatically made from amino acids. They have amino groups in them, but no longer have carboxyl groups and are therefore no longer classified as amino acids. Members of this group include serotonin, histamine and the catecholamines (dopamine, norepinephrine/noradrenaline and epinephrine/adrenaline). Serotonin (which is the basis of the serotonergic system) is made from tryptophan and has its own specific receptors. Dopamine is part of its own system, the dopaminergic system, which has dopamine receptors. Norepinephrine and epinephrine belong to the adrenergic neurotransmitter system. The two molecules are very similar and bind to the same receptors, which are referred to as alpha and beta receptors. The chemical epinephrine (epi- = “on”; “-nephrine” = kidney) is also known as adrenaline (renal = “kidney”), and norepinephrine is sometimes referred to as noradrenaline. The adrenal gland produces epinephrine and norepinephrine to be released into the blood stream as hormones. Once released into the synatpic cleft, all of these neurotransmitters are transported back into their respective presynaptic end bulb for repackaging and re-release.
The biogenic amines have mixed effects. For example, the dopamine receptors that are classified as D1 receptors are excitatory whereas D2-type receptors are inhibitory. Biogenic amine receptors can have even more complex effects because some may not directly affect the membrane potential, but rather have an effect on gene transcription or other metabolic processes in the neuron. The characteristics of the various neurotransmitter systems presented in this section are organized in Table 12.3 .
Large Molecule Neurotransmitters: Neuropeptides
A neuropeptide is a neurotransmitter molecule made up of chains of amino acids connected by peptide bonds; essentially a mini-protein. Neuropeptides are often released at synapses in combination with another neurotransmitter, and they often act as hormones in other systems of the body, such as oxytocin, vasoactive intestinal peptide (VIP) or substance P. In addition, sometimes neuropeptides contain other neuropeptides within them! In the case of endorphins, once released, endorphins are cleaved by extracellular enzymes to produce enkephalins, both of which bind to opiod receptors to modulate pain perception in the brain.
The characteristics of the various neurotransmitter systems presented in this section are organized in Table 12.3 .
Receptor Mechanism of Action
The important thing to remember about neurotransmitters, and signaling chemicals in general, is that the effect is entirely dependent on the receptor. Neurotransmitters bind to one of two classes of receptors at the cell surface, ionotropic or metabotropic ( Figure 12.4.2 ). Ionotropic receptors are ligand-gated ion channels, such as the nicotinic receptor for acetylcholine or the glycine receptor. A metabotropic receptor involves a complex of proteins that result in metabolic changes within the cell. The receptor complex includes the transmembrane receptor protein, a G protein, and an effector protein. The neurotransmitter, referred to as the first messenger, binds to the receptor protein on the extracellular surface of the cell, and the intracellular side of the protein initiates activity of the G protein. The G protein is a guanosine triphosphate (GTP) hydrolase that physically moves from the receptor protein to the effector protein to activate the latter. An effector protein is an enzyme that catalyzes the generation of a new molecule, which acts as the intracellular mediator, or the second messenger.
Different receptors use different second messengers. Two common examples of second messengers are cyclic adenosine monophosphate (cAMP) and inositol triphosphate (IP 3 ). The enzyme adenylate cyclase (an example of an effector protein) makes cAMP, and phospholipase C is the enzyme that makes IP 3 . Second messengers, after they are produced by the effector protein, cause metabolic changes within the cell. These changes are most likely the activation of other enzymes in the cell. In neurons, they often modify ion channels, either opening or closing them. These enzymes can also cause changes in the cell, such as the activation of genes in the nucleus, and therefore the increased synthesis of proteins. In neurons, these kinds of changes are often the basis of stronger connections between cells at the synapse and may be the basis of learning and memory.
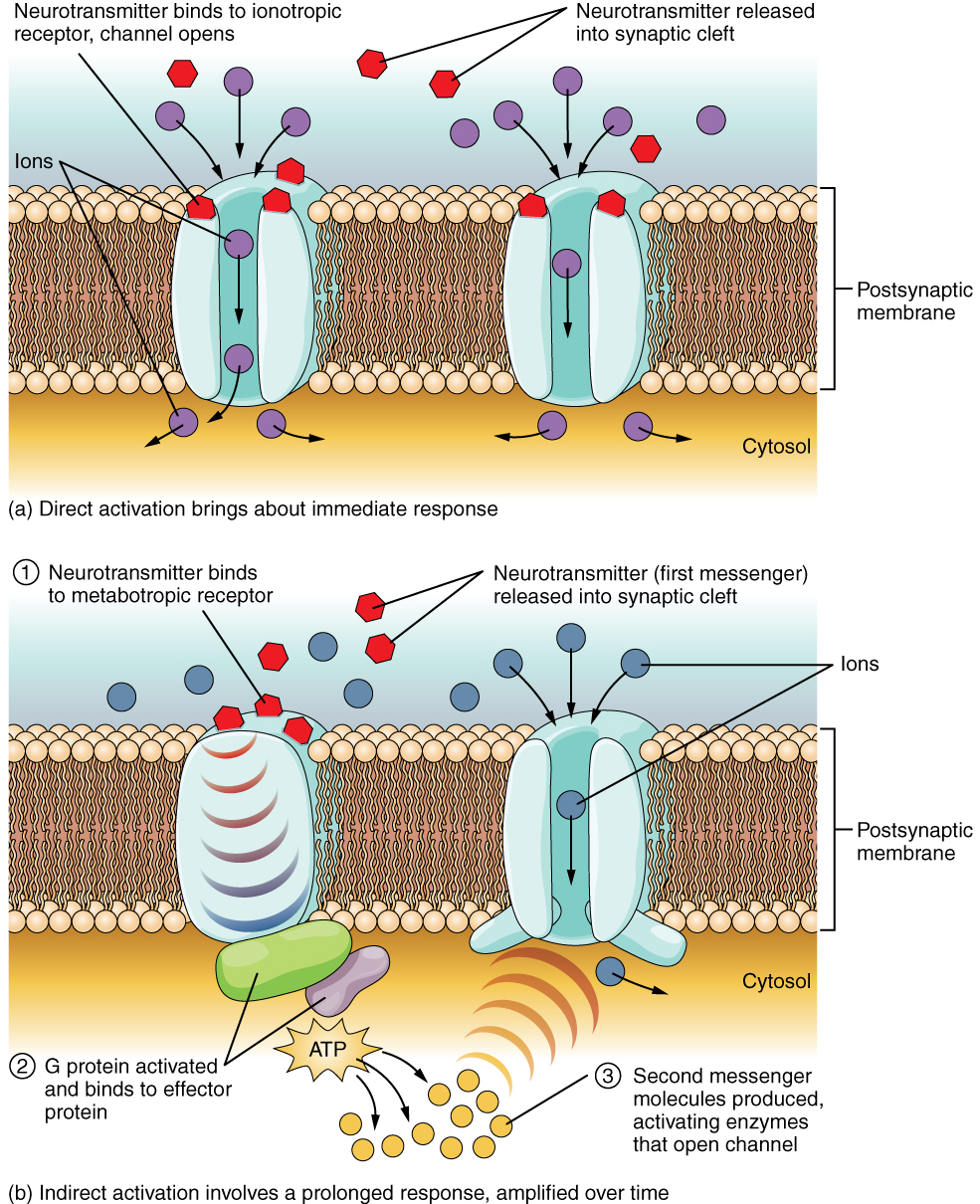
External Website

Watch this video to learn about the release of a neurotransmitter. The action potential reaches the end of the axon, called the axon terminal, and a chemical signal is released to tell the target cell to do something—either to initiate a new action potential, or to suppress that activity. In a very short space, the electrical signal of the action potential is changed into the chemical signal of a neurotransmitter and then back to electrical changes in the target cell membrane. What is the importance of voltage-gated calcium channels in the release of neurotransmitters?
Local changes in the membrane potential away from resting levels are called graded potentials and are usually associated with opening gated channels on the membrane a neuron. The type and amount of change in the membrane potential is determined by the ion that crosses the membrane, how many ions cross and for how long. Graded potentials can be of two sorts, either they are depolarizing (above resting membrane potential) or hyperpolarizing (below resting membrane potential) ( Figure 12.4.3 ). Depolarizing graded potentials are often the result of Na + or Ca 2+ entering the cell. Both of these ions have higher concentrations outside the cell than inside; because they have a positive charge, when they move into the cell the membrane becomes less negative inside relative to the outside. Hyperpolarizing graded potentials can be caused by K + leaving the cell or Cl – entering the cell. The membrane becomes more negative if a positive charge moves out of a cell or if a negative charge enters the cell. Graded potentials are transient and are dissipated as they move away from the site of the initial stimulus.
When ion channels are left open longer or more channels are opened (for the same ion), the stimulus affecting a neuron is bigger. A “bigger stimulus” occurs due to a more painful stimulus, a heavier load, a brighter light etc. These larger stimuli induce larger graded potentials of longer duration in neurons and can be either depolarizing or hyperpolarizing.
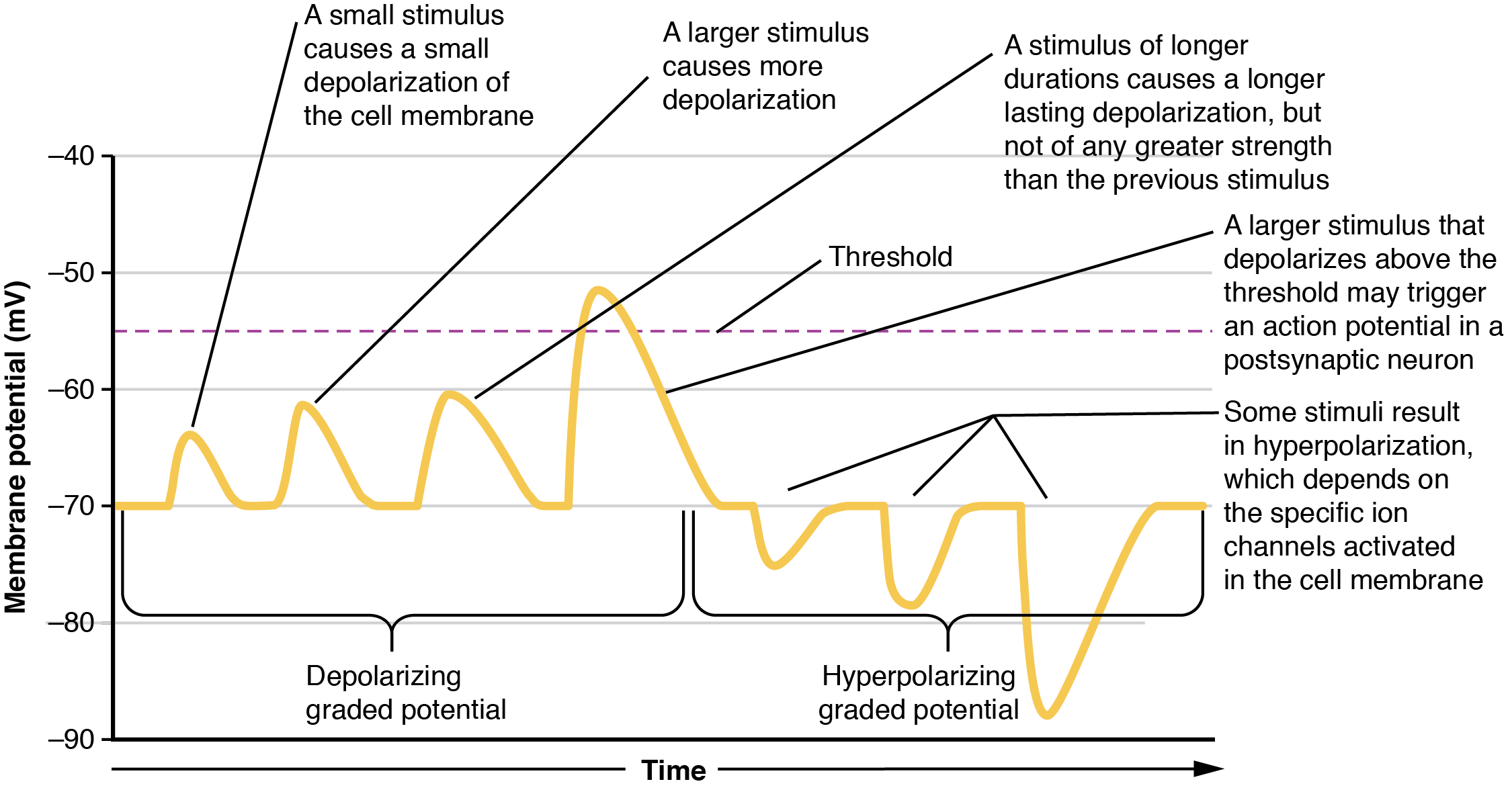
For the unipolar cells of sensory neurons—both those with free nerve endings and those within encapsulations—graded potentials develop in the dendrites and influence the generation of an action potential in the axon of the same cell. This is called a generator potential . For other sensory receptor cells which are not neurons, such as taste cells or photoreceptors of the retina, graded potentials in receptor cell membranes result in the release of neurotransmitters at synapses with sensory neurons. This is called a receptor potential, and we will consider this type of graded potential during a discussion of the special senses.
A postsynaptic potential (PSP) is the graded potential in the dendrites or cell body of a neuron that is receiving synapses from other cells. Postsynaptic potentials can be depolarizing or hyperpolarizing. Depolarization in a postsynaptic potential is called an excitatory postsynaptic potential (EPSP) because it causes the membrane potential to move toward threshold. Hyperpolarization in a postsynaptic potential is an inhibitory postsynaptic potential (IPSP) because it causes the membrane potential to move away from threshold.
All types of graded potentials will result in small changes (either depolarization or hyperpolarization) in the voltage of a membrane. These changes can lead to the neuron reaching threshold if the changes add together, or summate . The combined effects of different types of graded potentials are illustrated in Figure 12.4.4 . If the total change in voltage that reaches the initial segment (or trigger zone) is a positive 15 mV, meaning that the membrane depolarizes from -70 mV (resting membrane potential) to -55 mV (threshold), then the graded potentials will result in the initiation of an action potential.
Graded potentials summate at a specific location at the beginning of the axon to initiate the action potential, namely the initial segment. For sensory neurons, the initial segment is directly adjacent to the dendritic endings (since the cell body is located more proximally). For all other neurons, the initial segment of the axon is found at the axon hillock and it is where summation takes place. These locations have a high density of voltage-gated Na + channels that initiate the depolarizing phase of the action potential and is often referred as the trigger zone.
Summation can be spatial or temporal, meaning it can be the result of multiple graded potentials occurring simultaneously at different locations on the neuron (spatial), or all at the same place but in rapid succession (temporal). Spatial and temporal summation can act together, as well. Since graded potentials dissipated with distance and time, summation is the total change in voltage due to all spatial and temporal graded potentials that reach the trigger zone or initial segment at each moment.
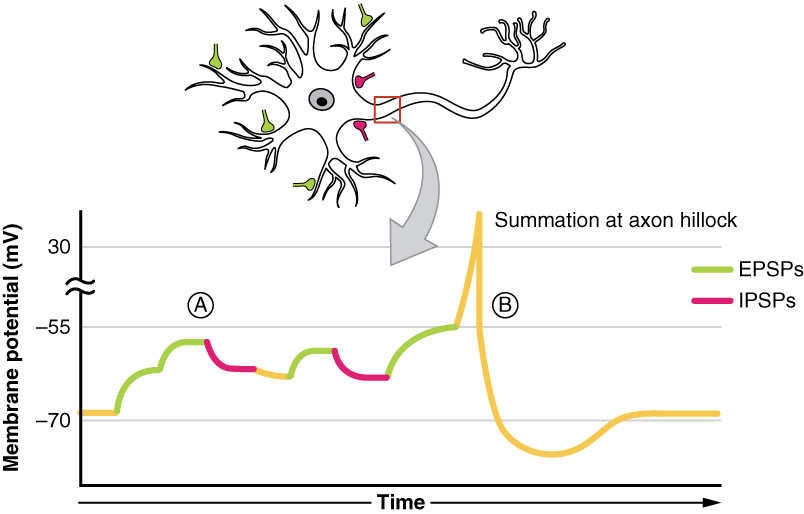
Watch this video to learn about summation. The process of converting electrical signals to chemical signals and back requires subtle changes that can result in transient increases or decreases in membrane voltage. To cause a lasting change in the target cell, multiple signals are usually added together, or summated. Does spatial summation have to happen all at once, or can the separate signals arrive on the postsynaptic neuron at slightly different times? Explain your answer.
The underlying cause of some neurodegenerative diseases, such as Alzheimer’s and Parkinson’s, appears to be related to proteins—specifically, to proteins behaving badly. One of the strongest theories of what causes Alzheimer’s disease is based on the accumulation of beta-amyloid plaques, dense conglomerations of a protein that is not functioning correctly. Parkinson’s disease is linked to an increase in a protein known as alpha-synuclein that is toxic to the cells of the substantia nigra nucleus in the midbrain.
For proteins to function correctly, they are dependent on their three-dimensional shape. The linear sequence of amino acids folds into a three-dimensional shape that is based on the interactions between and among those amino acids. When the folding is disturbed, and proteins take on a different shape, they stop functioning correctly. But the disease is not necessarily the result of functional loss of these proteins; rather, these altered proteins start to accumulate and may become toxic. For example, in Alzheimer’s, the hallmark of the disease is the accumulation of these amyloid plaques in the cerebral cortex. The term coined to describe this sort of disease is “proteopathy” and it includes other diseases. Creutzfeld-Jacob disease, the human variant of the prion disease known as mad cow disease in the bovine, also involves the accumulation of amyloid plaques, similar to Alzheimer’s. Diseases of other organ systems can fall into this group as well, such as cystic fibrosis or type 2 diabetes. Recognizing the relationship between these diseases has suggested new therapeutic possibilities. Interfering with the accumulation of the proteins, and possibly as early as their original production within the cell, may unlock new ways to alleviate these devastating diseases.
Chapter Review
The basis of the electrical signal within a neuron is the action potential that propagates down the axon. For a neuron to generate an action potential, it needs to receive input from another source, either another neuron or a sensory stimulus. That input will result in opening ion channels in the neuron, resulting in a graded potential based on the strength of the stimulus. Graded potentials can be depolarizing or hyperpolarizing and can summate to affect the probability of the neuron reaching threshold at the initial segment or trigger zone. Graded potentials produced by interactions between neurons at synapses are called postsynaptic potentials (PSPs). A depolarizing graded potential at a synapse is called an excitatory PSP, and a hyperpolarizing graded potential at a synapse is called an inhibitory PSP.
Synapses are the contacts between neurons, which can either be chemical or electrical in nature. Chemical synapses are far more common. At a chemical synapse, neurotransmitter is released from the presynaptic element and diffuses across the synaptic cleft. The neurotransmitter binds to a receptor protein and causes a change in the postsynaptic membrane (the PSP). The neurotransmitter must be inactivated or removed from the synaptic cleft so that the stimulus is limited in time.
The particular characteristics of a synapse vary based on the neurotransmitter system produced by that neuron. The cholinergic system is found at the neuromuscular junction and in certain places within the nervous system. Amino acids, such as glutamate, glycine, and gamma-aminobutyric acid (GABA) are used as neurotransmitters. Other neurotransmitters are the result of amino acids being enzymatically changed, as in the biogenic amines, or being covalently bonded together, as in the neuropeptides.
Interactive Link Questions
A second signal from a separate presynaptic neuron can arrive slightly later, as long as it arrives before the first one dies off, or dissipates.
Watch this video to learn about the release of a neurotransmitter. The action potential reaches the end of the axon, called the axon terminal, and a chemical signal is released to tell the target cell to do something, either initiate a new action potential, or to suppress that activity. In a very short space, the electrical signal of the action potential is changed into the chemical signal of a neurotransmitter, and then back to electrical changes in the target cell membrane. What is the importance of voltage-gated calcium channels in the release of neurotransmitters?
The action potential depolarizes the cell membrane of the axon terminal, which contains the voltage-gated Ca 2+ channel. That voltage change opens the channel so that Ca 2+ can enter the axon terminal. Calcium ions make it possible for synaptic vesicles to release their contents through exocytosis.
Review Questions
Critical thinking questions.
1. If a postsynaptic cell has synapses from five different cells, and three cause EPSPs and two of them cause IPSPs, give an example of a series of depolarizations and hyperpolarizations that would result in the neuron reaching threshold.
2. Why is the receptor the important element determining the effect a neurotransmitter has on a target cell?
Answers for Critical Thinking Questions
- EPSP1 = +5 mV, EPSP2 = +7 mV, EPSP 3 = +10 mV, IPSP1 = -4 mV, IPSP2 = -3 mV. 5 + 7 + 10 – 4 – 3 = +15 mV.
- Different neurotransmitters have different receptors. Thus, the type of receptor in the postsynaptic cell is what determines which ion channels open. Acetylcholine binding to the nicotinic receptor causes cations to cross the membrane. GABA binding to its receptor causes the anion chloride to cross the membrane.
This work, Anatomy & Physiology, is adapted from Anatomy & Physiology by OpenStax , licensed under CC BY . This edition, with revised content and artwork, is licensed under CC BY-SA except where otherwise noted.
Images, from Anatomy & Physiology by OpenStax , are licensed under CC BY except where otherwise noted.
Access the original for free at https://openstax.org/books/anatomy-and-physiology/pages/1-introduction .
Anatomy & Physiology Copyright © 2019 by Lindsay M. Biga, Staci Bronson, Sierra Dawson, Amy Harwell, Robin Hopkins, Joel Kaufmann, Mike LeMaster, Philip Matern, Katie Morrison-Graham, Kristen Oja, Devon Quick, Jon Runyeon, OSU OERU, and OpenStax is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License , except where otherwise noted.
- Brain Development
- Childhood & Adolescence
- Diet & Lifestyle
- Emotions, Stress & Anxiety
- Learning & Memory
- Thinking & Awareness
- Alzheimer's & Dementia
- Childhood Disorders
- Immune System Disorders
- Mental Health
- Neurodegenerative Disorders
- Infectious Disease
- Neurological Disorders A-Z
- Body Systems
- Cells & Circuits
- Genes & Molecules
- The Arts & the Brain
- Law, Economics & Ethics
- Neuroscience in the News
- Supporting Research
- Tech & the Brain
- Animals in Research
- BRAIN Initiative
- Meet the Researcher
- Neuro-technologies
- Tools & Techniques
Core Concepts
- For Educators
- Ask an Expert
- The Brain Facts Book

How Neurons Communicate
- Sensory stimuli are converted to electrical signals.
- Action potentials are electrical signals carried along neurons.
- Synapses are chemical or electrical junctions that allow electrical signals to pass from neurons to other cells.
- Electrical signals in muscles cause contraction and movement.
- Changes in the amount of activity at a synapse can enhance or reduce its function.
- Communication between neurons is strengthened or weakened by an individual's activities, such as exercise, stress, and drug use.
- All perceptions, thoughts, and behaviors result from combinations of signals among neurons.
- Wellcome Trust

- BrainFacts/SfN

An interactive brain map that you can rotate in a three-dimensional space.
A beginner's guide to the brain and nervous system.
Image of the Week
Check out the Image of the Week Archive.
BrainFacts Book
Download a copy of the newest edition of the book, Brain Facts: A Primer on the Brain and Nervous System.

SUPPORTING PARTNERS
- Accessibility Policy
- Terms and Conditions
- Manage Cookies
Some pages on this website provide links that require Adobe Reader to view.
A Picture-Perfect Look at How Electrical Activity Travels through the Brain
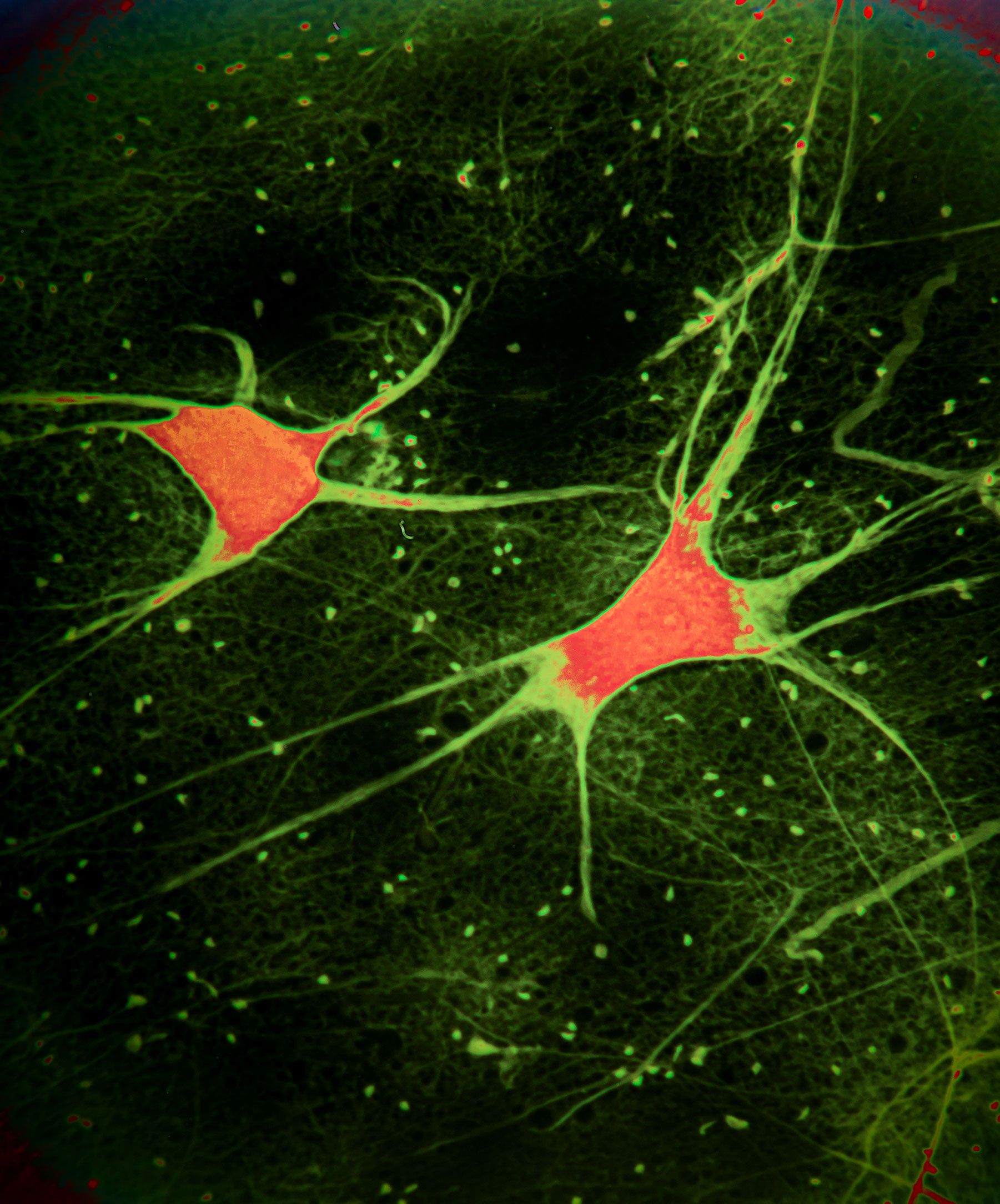
Photo by jxfzsy/iStock
New imaging technique developed by BU, MIT researchers can detect more individual brain cells firing in the brain than ever before
Anne trafton.
Brain cells function using rapid electrical impulses, a process that underlies our thoughts, behavior, and perception of the world. Yet, for a long time, it’s been challenging for scientists to see exactly how individual neurons work together in larger circuits.
Now, a new technique reported in Nature finally gives the clearest picture ever of brain cell activity. Using a voltage-sensing molecule that fluorescently lights up when brain cells are electrically active, researchers at Boston University and the Massachusetts Institute of Technology have shown that they can see the activity of many more individual neurons than before as they fire inside the brains of mice.
With the new voltage sensor, it is also possible to measure very small fluctuations in activity that occur even when a neuron is not firing a big spike in electrical activity. This could help neuroscientists study how small fluctuations impact a neuron’s overall behavior, which has previously been very difficult to do in living brains, says paper co–corresponding author Xue Han , a BU College of Engineering associate professor of biomedical engineering, describing the advance.
This technique can be performed using a simple light microscope, and it could allow neuroscientists to link firing of certain cell groups to specific behaviors, says co–corresponding author Edward Boyden of MIT. “If you want to study a behavior, or a disease, you need to image the activity of populations of neurons because they work together in a network.”
Until now, it’s been possible to measure the electrical activity of neurons by inserting an electrode into the brain, but this technique is labor-intensive and typically only allows researchers to record activity from one neuron at a time. Multielectrode arrays can allow monitoring of electrical activity from many neurons at once, but it’s impossible to record the densely packed activities of all neurons within a piece of brain tissue. A technique called calcium imaging does allow such dense sampling, but it measures calcium, which is an indirect and slow measure of neural electrical activity.
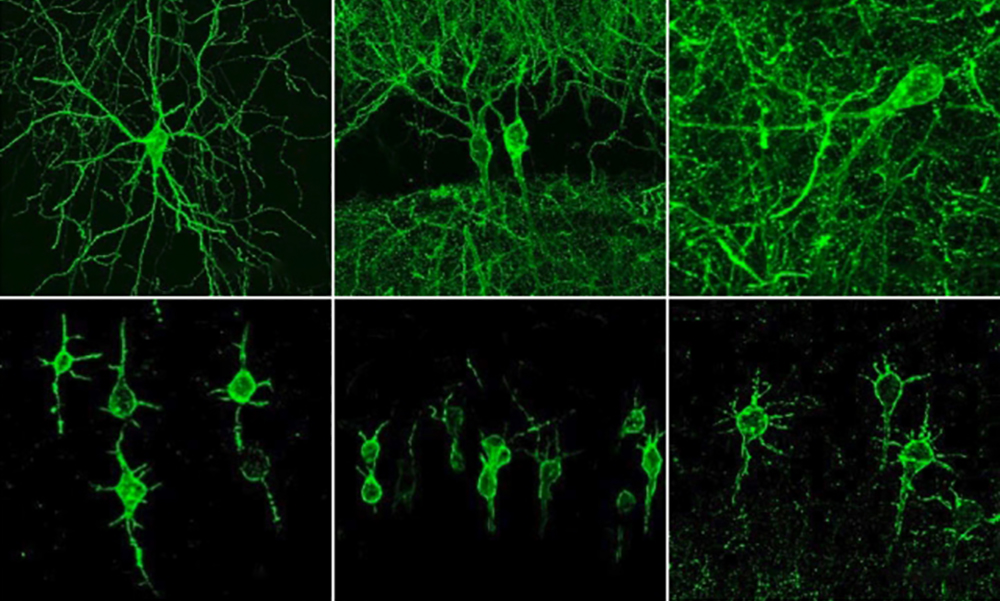
In 2018, Boyden’s team first developed an alternative way to monitor electrical activity by labeling neurons with a fluorescent probe. They engineered a molecule called Archon1 that can be genetically inserted into neurons, where it becomes embedded in the cell membrane. When a neuron’s electrical activity increases, the molecule becomes brighter, and this fluorescence can be seen with a standard light microscope. The researchers showed that they could use the molecule to image electrical activity in the brains of transparent worms and zebrafish embryos, and also in mouse brain slices. But for the new study, they wanted to try to use it in living, awake mice as they engaged in a specific behavior.
To do that, the researchers needed to modify the probe so that it would go to a more specific region of the neuron’s cell membrane, because when the molecule inserts itself throughout the entire cell membrane, the resulting images are blurry from the sprawling tendrils—called axons and dendrites—that extend outward from neurons like the arms of a starfish. The research team engineered a new version of Archon1 that sticks specifically to membranes of the central cell bodies of neurons, but not its outer axons or dendrites. They named this modified voltage-tracking molecule SomArchon.
“With SomArchon, you can see each cell as a distinct sphere,” Boyden says. “Rather than having one cell’s light blurring all its neighbors, each cell can speak by itself loudly and clearly, uncontaminated by its neighbors.”
To test how it worked in live mice, Boyden partnered with Han’s lab at BU. “My lab has been doing a lot of work on imaging single neurons inside the brain,” says Han. “We’ve used calcium imaging to look at a large number of neurons…but the timescale is very slow for calcium imaging. Voltage changes are all happening at the timescale of milliseconds. That’s why we have been trying to do voltage imaging.”
Genetically engineering live mice to have SomArchon in their brain cells, the research team imaged electrical activity in a part of the brain involved in planning movement, called the striatum, while the mice ran on a ball. They were able to monitor electrical activity in many neurons simultaneously and correlate each one’s activity with the mice’s movement. Some neurons’ activity went up when the mice were running, some went down, and others showed no significant change. Former BU graduate researcher Seth Bensussen, one of three co–first authors on the study, led the team’s brain imaging efforts, and BU research scientist Hua-an Tseng led the computational aspect of the work.
“Over the years, my lab has tried many different versions of voltage sensors, and none of them have worked in living mammalian brains until this one,” Han says. “With this technology, we can look at subtle changes in how voltage travels through brain cell membranes. A big part of my research is looking at the dynamics related to Parkinson’s disease and deep brain stimulation. These voltage sensors will allow us to study how individual neurons communicate to generate larger neurological phenomena. Ultimately, it could guide in the design of new therapies to treat neurodegenerative diseases.”
The team also showed that the voltage sensors can be combined with optogenetics, a technique that Han and Boyden pioneered over a decade ago, that allows researchers to turn brain cells on and off with laser light. In this case, the researchers activated certain neurons with light and then measured the resulting electrical activity in these neurons.
This work was supported by funding from the National Institutes of Health, the National Science Foundation, the Howard Hughes Medical Institute Simons Faculty Scholars Program, the Human Frontier Science Program, the US Army Research Office, the Grainger Foundation, the Pew Foundation, and Boston University Biomedical Engineering Department.
This story was adapted from an MIT article originally written by Anne Trafton.
Explore Related Topics:
- Neurotechnology
- Share this story
- 5 Comments Add
Anne Trafton Profile
Comments & Discussion
Boston University moderates comments to facilitate an informed, substantive, civil conversation. Abusive, profane, self-promotional, misleading, incoherent or off-topic comments will be rejected. Moderators are staffed during regular business hours (EST) and can only accept comments written in English. Statistics or facts must include a citation or a link to the citation.
There are 5 comments on A Picture-Perfect Look at How Electrical Activity Travels through the Brain
I’m currently working on a project about the eradication of epileptic seizures. So my question is where and how do epileptic seizures start in the brain? What causes certain individuals to suffer from epileptic seizures and some not? What is the electrical activity like when some one is undergoing an epileptic seizure
If the brain is electrical in nature? (And we know that electricity cannot be destroyed), what then happens to that energy once our bodies have completely broken down after death?
I would think of it like an alkeline battery either buried in the ground (slow discharge) or burnt in a fire (converted to heat).
According to Dr. Leroy Little Bear, professor emeritus at the University of Lethbridge, and member of the Blackfoot tribe in Canada, while physicists were contemplating the particle/wave duality of electrons and matter in general, Little Bear asserted that in Native Science, it’s all waves, energy waves, and those waves are spirit. When we die, those particular wave patterns dissipate and take on a different configuration. But you are still you. YouTube has several of his lectures. I was able to attend the one in 2011 at the Heard Museum at Phoenix. It was part of a series of Native American speakers sponsored by Arizona State University. This particular one compared Western and Native Science, which Little Bear said were becoming closer to one another through discoveries in quantum physics and string theory. You might want to check it out.
Ok. But now the real question, where does the electricity come from? Does the pregnant person’s brain gain more electricity to give to the child?
Post a comment. Cancel reply
Your email address will not be published. Required fields are marked *
Latest from The Brink
What a southern plantation’s paper trail can reveal about the lasting legacies of slavery, why are kids struggling with anxiety more than ever, getting their hands dirty in the lab—and in the charles river, 2024 ignition awards aim to bring bu science and tech to market, bu team wins major national science foundation grant to help phd students attack climate change, liberation through rhythm: bu ethnomusicologist studies history and present of african beats, oxygen produced in the deep sea raises questions about extraterrestrial life, the histories of enslaved people were written by slavers. a bu researcher is working to change that, making mri more globally accessible: how metamaterials offer affordable, high-impact solutions, “i love this work, but it’s killing me”: the unique toll of being a spiritual leader today, feeling the heat researchers say heat waves will put more older adults in danger, what the history of boston’s harbor can teach us about its uncertain future, eng’s mark grinstaff one of six researchers to receive nsf trailblazer engineering impact awards, how do we solve america’s affordable housing crisis bu research helps inspire a federal bill that suggests answers, missile defense won’t save us from growing nuclear arsenals, this ai software can make diagnosing dementia easier and faster for doctors, suicide now the primary cause of death among active duty us soldiers, state laws banning abortion linked to increases in mental health issues, scuba diving safely for marine biology research, heat waves are scorching boston, but are some neighborhoods hotter than others.
35.2 How Neurons Communicate
Learning objectives.
By the end of this section, you will be able to do the following:
- Describe the basis of the resting membrane potential
- Explain the stages of an action potential and how action potentials are propagated
- Explain the similarities and differences between chemical and electrical synapses
- Describe long-term potentiation and long-term depression
All functions performed by the nervous system—from a simple motor reflex to more advanced functions like making a memory or a decision—require neurons to communicate with one another. While humans use words and body language to communicate, neurons use electrical and chemical signals. Just like a person in a committee, one neuron usually receives and synthesizes messages from multiple other neurons before “making the decision” to send the message on to other neurons.

Nerve Impulse Transmission within a Neuron
For the nervous system to function, neurons must be able to send and receive signals. These signals are possible because each neuron has a charged cellular membrane (a voltage difference between the inside and the outside), and the charge of this membrane can change in response to neurotransmitter molecules released from other neurons and environmental stimuli. To understand how neurons communicate, one must first understand the basis of the baseline or ‘resting’ membrane charge.
Neuronal Charged Membranes
The lipid bilayer membrane that surrounds a neuron is impermeable to charged molecules or ions. To enter or exit the neuron, ions must pass through special proteins called ion channels that span the membrane. Ion channels have different configurations: open, closed, and inactive, as illustrated in Figure 35.9 . Some ion channels need to be activated in order to open and allow ions to pass into or out of the cell. These ion channels are sensitive to the environment and can change their shape accordingly. Ion channels that change their structure in response to voltage changes are called voltage-gated ion channels. Voltage-gated ion channels regulate the relative concentrations of different ions inside and outside the cell. The difference in total charge between the inside and outside of the cell is called the membrane potential .
Link to Learning
This video discusses the basis of the resting membrane potential.
Resting Membrane Potential
A neuron at rest is negatively charged: the inside of a cell is approximately 70 millivolts more negative than the outside (−70 mV, note that this number varies by neuron type and by species). This voltage is called the resting membrane potential; it is caused by differences in the concentrations of ions inside and outside the cell. If the membrane were equally permeable to all ions, each type of ion would flow across the membrane and the system would reach equilibrium. Because ions cannot simply cross the membrane at will, there are different concentrations of several ions inside and outside the cell, as shown in Table 35.1 . The difference in the number of positively charged potassium ions (K + ) inside and outside the cell dominates the resting membrane potential ( Figure 35.10 ). When the membrane is at rest, K + ions accumulate inside the cell due to the activity of the Na/K pump, driving both ions against their concentration gradient. The negative resting membrane potential is created and maintained by increasing the concentration of cations outside the cell (in the extracellular fluid) relative to inside the cell (in the cytoplasm). The negative charge within the cell is created by the cell membrane being more permeable to potassium ion movement than sodium ion movement. In neurons, potassium ions are maintained at high concentrations within the cell while sodium ions are maintained at high concentrations outside of the cell. The cell possesses potassium and sodium leakage channels that allow the two cations to diffuse down their concentration gradient. However, the neurons have far more potassium leakage channels than sodium leakage channels. Therefore, potassium diffuses out of the cell at a much faster rate than sodium leaks in. Because more cations are leaving the cell than are entering, this causes the interior of the cell to be negatively charged relative to the outside of the cell. The actions of the sodium potassium pump help to maintain the resting potential, once established. Recall that sodium potassium pumps brings two K + ions into the cell while removing three Na + ions per ATP consumed. As more cations are expelled from the cell than taken in, the inside of the cell remains negatively charged relative to the extracellular fluid. It should be noted that chloride ions (Cl – ) tend to accumulate outside of the cell because they are repelled by negatively-charged proteins within the cytoplasm.
Action Potential
A neuron can receive input from other neurons and, if this input is strong enough, send the signal to downstream neurons. Transmission of a signal between neurons is generally carried by a chemical called a neurotransmitter. Transmission of a signal within a neuron (from dendrite to axon terminal) is carried by a brief reversal of the resting membrane potential called an action potential . When neurotransmitter molecules bind to receptors located on a neuron’s dendrites, ion channels open. At excitatory synapses, this opening allows positive ions to enter the neuron and results in depolarization of the membrane—a decrease in the difference in voltage between the inside and outside of the neuron. A stimulus from a sensory cell or another neuron depolarizes the target neuron to its threshold potential (-55 mV). Na + channels in the axon hillock open, allowing positive ions to enter the cell ( Figure 35.10 and Figure 35.11 ). Once the sodium channels open, the neuron completely depolarizes to a membrane potential of about +40 mV. Action potentials are considered an "all-or nothing" event, in that, once the threshold potential is reached, the neuron always completely depolarizes. Once depolarization is complete, the cell must now "reset" its membrane voltage back to the resting potential. To accomplish this, the Na + channels close and cannot be opened. This begins the neuron's refractory period , in which it cannot produce another action potential because its sodium channels will not open. At the same time, voltage-gated K + channels open, allowing K + to leave the cell. As K + ions leave the cell, the membrane potential once again becomes negative and repolarizes. The diffusion of K+ out of the cell actually continues for a short period of time past the time of the achievement of the resting potential, and the membrane hyperpolarizes, in that the membrane potential becomes more negative than the cell's normal resting potential. This is the result of the slow closing of the K + channels. At this point, the sodium channels will return to their resting state, meaning they are ready to open again if the membrane potential again exceeds the threshold potential. Eventually all the K+ channels close, and the cell returns back to its resting membrane potential.
Visual Connection
Potassium channel blockers, such as amiodarone and procainamide, which are used to treat abnormal electrical activity in the heart, called cardiac dysrhythmia, impede the movement of K + through voltage-gated K + channels. Which part of the action potential would you expect potassium channels to affect?
This video presents an overview of action potential.
Myelin and the Propagation of the Action Potential
For an action potential to communicate information to another neuron, it must travel along the axon and reach the axon terminals where it can initiate neurotransmitter release. The speed of conduction of an action potential along an axon is influenced by both the diameter of the axon and the axon’s resistance to current leak. Myelin acts as an insulator that prevents current from leaving the axon; this increases the speed of action potential conduction. In demyelinating diseases like multiple sclerosis, action potential conduction slows because current leaks from previously insulated axon areas. The nodes of Ranvier, illustrated in Figure 35.13 are gaps in the myelin sheath along the axon. These unmyelinated spaces are about one micrometer long and contain voltage-gated Na + and K + channels. Flow of ions through these channels, particularly the Na + channels, regenerates the action potential over and over again along the axon. This ‘jumping’ of the action potential from one node to the next is called saltatory conduction . If nodes of Ranvier were not present along an axon, the action potential would propagate very slowly since Na + and K + channels would have to continuously regenerate action potentials at every point along the axon instead of at specific points. Nodes of Ranvier also save energy for the neuron since the channels only need to be present at the nodes and not along the entire axon.
Synaptic Transmission
The synapse or “gap” is the place where information is transmitted from one neuron to another. Synapses usually form between axon terminals and dendritic spines, but this is not universally true. There are also axon-to-axon, dendrite-to-dendrite, and axon-to-cell body synapses. The neuron transmitting the signal is called the presynaptic neuron, and the neuron receiving the signal is called the postsynaptic neuron. Note that these designations are relative to a particular synapse—most neurons are both presynaptic and postsynaptic. There are two types of synapses: chemical and electrical.
Chemical Synapse
When an action potential reaches the axon terminal it depolarizes the membrane and opens voltage-gated Na + channels. Na + ions enter the cell, further depolarizing the presynaptic membrane. This depolarization causes voltage-gated Ca 2+ channels to open. Calcium ions entering the cell initiate a signaling cascade that causes small membrane-bound vesicles, called synaptic vesicles , containing neurotransmitter molecules to fuse with the presynaptic membrane. Synaptic vesicles are shown in Figure 35.14 , which is an image from a scanning electron microscope.
Fusion of a vesicle with the presynaptic membrane causes neurotransmitter to be released into the synaptic cleft , the extracellular space between the presynaptic and postsynaptic membranes, as illustrated in Figure 35.15 . The neurotransmitter diffuses across the synaptic cleft and binds to receptor proteins on the postsynaptic membrane.
The binding of a specific neurotransmitter causes particular ion channels, in this case ligand-gated channels, on the postsynaptic membrane to open. Neurotransmitters can either have excitatory or inhibitory effects on the postsynaptic membrane. For example, when acetylcholine is released at the synapse between a nerve and muscle (called the neuromuscular junction) by a presynaptic neuron, it causes postsynaptic Na + channels to open. Na + enters the postsynaptic cell and causes the postsynaptic membrane to depolarize. This depolarization is called an excitatory postsynaptic potential (EPSP) and makes the postsynaptic neuron more likely to fire an action potential. Release of neurotransmitter at inhibitory synapses causes inhibitory postsynaptic potentials (IPSPs) , a hyperpolarization of the presynaptic membrane. For example, when the neurotransmitter GABA (gamma-aminobutyric acid) is released from a presynaptic neuron, it binds to and opens Cl - channels. Cl - ions enter the cell and hyperpolarizes the membrane, making the neuron less likely to fire an action potential.
Once neurotransmission has occurred, the neurotransmitter must be removed from the synaptic cleft so the postsynaptic membrane can “reset” and be ready to receive another signal. This can be accomplished in three ways: the neurotransmitter can diffuse away from the synaptic cleft, it can be degraded by enzymes in the synaptic cleft, or it can be recycled (sometimes called reuptake) by the presynaptic neuron. Several drugs act at this step of neurotransmission. For example, some drugs that are given to Alzheimer’s patients work by inhibiting acetylcholinesterase, the enzyme that degrades acetylcholine. This inhibition of the enzyme essentially increases neurotransmission at synapses that release acetylcholine. Once released, the acetylcholine stays in the cleft and can continually bind and unbind to postsynaptic receptors.
Electrical Synapse
While electrical synapses are fewer in number than chemical synapses, they are found in all nervous systems and play important and unique roles. The mode of neurotransmission in electrical synapses is quite different from that in chemical synapses. In an electrical synapse, the presynaptic and postsynaptic membranes are very close together and are actually physically connected by channel proteins forming gap junctions. Gap junctions allow current to pass directly from one cell to the next. In addition to the ions that carry this current, other molecules, such as ATP, can diffuse through the large gap junction pores.
There are key differences between chemical and electrical synapses. Because chemical synapses depend on the release of neurotransmitter molecules from synaptic vesicles to pass on their signal, there is an approximately one millisecond delay between when the axon potential reaches the presynaptic terminal and when the neurotransmitter leads to opening of postsynaptic ion channels. Additionally, this signaling is unidirectional. Signaling in electrical synapses, in contrast, is virtually instantaneous (which is important for synapses involved in key reflexes), and some electrical synapses are bidirectional. Electrical synapses are also more reliable as they are less likely to be blocked, and they are important for synchronizing the electrical activity of a group of neurons. For example, electrical synapses in the thalamus are thought to regulate slow-wave sleep, and disruption of these synapses can cause seizures.
Signal Summation
Sometimes a single EPSP is strong enough to induce an action potential in the postsynaptic neuron, but often multiple presynaptic inputs must create EPSPs around the same time for the postsynaptic neuron to be sufficiently depolarized to fire an action potential. This process is called summation and occurs at the axon hillock, as illustrated in Figure 35.16 . Additionally, one neuron often has inputs from many presynaptic neurons—some excitatory and some inhibitory—so IPSPs can cancel out EPSPs and vice versa. It is the net change in postsynaptic membrane voltage that determines whether the postsynaptic cell has reached its threshold of excitation needed to fire an action potential. Together, synaptic summation and the threshold for excitation act as a filter so that random “noise” in the system is not transmitted as important information.
Everyday Connection
Brain-computer interface.
Amyotrophic lateral sclerosis (ALS, also called Lou Gehrig’s Disease) is a neurological disease characterized by the degeneration of the motor neurons that control voluntary movements. The disease begins with muscle weakening and lack of coordination and eventually destroys the neurons that control speech, breathing, and swallowing; in the end, the disease can lead to paralysis. At that point, patients require assistance from machines to be able to breathe and to communicate. Several special technologies have been developed to allow “locked-in” patients to communicate with the rest of the world. One technology, for example, allows patients to type out sentences by twitching their cheek. These sentences can then be read aloud by a computer.
A relatively new line of research for helping paralyzed patients, including those with ALS, to communicate and retain a degree of self-sufficiency is called brain-computer interface (BCI) technology and is illustrated in Figure 35.17 . This technology sounds like something out of science fiction: it allows paralyzed patients to control a computer using only their thoughts. There are several forms of BCI. Some forms use EEG recordings from electrodes taped onto the skull. These recordings contain information from large populations of neurons that can be decoded by a computer. Other forms of BCI require the implantation of an array of electrodes smaller than a postage stamp in the arm and hand area of the motor cortex. This form of BCI, while more invasive, is very powerful as each electrode can record actual action potentials from one or more neurons. These signals are then sent to a computer, which has been trained to decode the signal and feed it to a tool—such as a cursor on a computer screen. This means that a patient with ALS can use e-mail, read the Internet, and communicate with others by thinking of moving their hand or arm (even though the paralyzed patient cannot make that bodily movement). Recent advances have allowed a paralyzed locked-in patient who suffered a stroke 15 years ago to control a robotic arm and even to feed herself coffee using BCI technology.
Despite the amazing advancements in BCI technology, it also has limitations. The technology can require many hours of training and long periods of intense concentration for the patient; it can also require brain surgery to implant the devices.
Watch this video in which a paralyzed woman uses a brain-controlled robotic arm to bring a drink to her mouth, among other images of brain-computer interface technology in action.
Synaptic Plasticity
Synapses are not static structures. They can be weakened or strengthened. They can be broken, and new synapses can be made. Synaptic plasticity allows for these changes, which are all needed for a functioning nervous system. In fact, synaptic plasticity is the basis of learning and memory. Two processes in particular, long-term potentiation (LTP) and long-term depression (LTD) are important forms of synaptic plasticity that occur in synapses in the hippocampus, a brain region that is involved in storing memories.
Long-term Potentiation (LTP)
Long-term potentiation (LTP) is a persistent strengthening of a synaptic connection. LTP is based on the Hebbian principle: cells that fire together wire together. There are various mechanisms, none fully understood, behind the synaptic strengthening seen with LTP. One known mechanism involves a type of postsynaptic glutamate receptor, called NMDA (N-Methyl-D-aspartate) receptors, shown in Figure 35.18 . These receptors are normally blocked by magnesium ions; however, when the postsynaptic neuron is depolarized by multiple presynaptic inputs in quick succession (either from one neuron or multiple neurons), the magnesium ions are forced out allowing Ca ions to pass into the postsynaptic cell. Next, Ca 2+ ions entering the cell initiate a signaling cascade that causes a different type of glutamate receptor, called AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) receptors, to be inserted into the postsynaptic membrane, since activated AMPA receptors allow positive ions to enter the cell. So, the next time glutamate is released from the presynaptic membrane, it will have a larger excitatory effect (EPSP) on the postsynaptic cell because the binding of glutamate to these AMPA receptors will allow more positive ions into the cell. The insertion of additional AMPA receptors strengthens the synapse and means that the postsynaptic neuron is more likely to fire in response to presynaptic neurotransmitter release. Some drugs of abuse co-opt the LTP pathway, and this synaptic strengthening can lead to addiction.
Long-term Depression (LTD)
Long-term depression (LTD) is essentially the reverse of LTP: it is a long-term weakening of a synaptic connection. One mechanism known to cause LTD also involves AMPA receptors. In this situation, calcium that enters through NMDA receptors initiates a different signaling cascade, which results in the removal of AMPA receptors from the postsynaptic membrane, as illustrated in Figure 35.18 . The decrease in AMPA receptors in the membrane makes the postsynaptic neuron less responsive to glutamate released from the presynaptic neuron. While it may seem counterintuitive, LTD may be just as important for learning and memory as LTP. The weakening and pruning of unused synapses allows for unimportant connections to be lost and makes the synapses that have undergone LTP that much stronger by comparison.
This book may not be used in the training of large language models or otherwise be ingested into large language models or generative AI offerings without OpenStax's permission.
Want to cite, share, or modify this book? This book uses the Creative Commons Attribution License and you must attribute OpenStax.
Access for free at https://openstax.org/books/biology-2e/pages/1-introduction
- Authors: Mary Ann Clark, Matthew Douglas, Jung Choi
- Publisher/website: OpenStax
- Book title: Biology 2e
- Publication date: Mar 28, 2018
- Location: Houston, Texas
- Book URL: https://openstax.org/books/biology-2e/pages/1-introduction
- Section URL: https://openstax.org/books/biology-2e/pages/35-2-how-neurons-communicate
© Jul 10, 2024 OpenStax. Textbook content produced by OpenStax is licensed under a Creative Commons Attribution License . The OpenStax name, OpenStax logo, OpenStax book covers, OpenStax CNX name, and OpenStax CNX logo are not subject to the Creative Commons license and may not be reproduced without the prior and express written consent of Rice University.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Alcohol Res Health
- v.31(3); 2008

Communication Networks in the Brain
Nerve cells (i.e., neurons) communicate via a combination of electrical and chemical signals. Within the neuron, electrical signals driven by charged particles allow rapid conduction from one end of the cell to the other. Communication between neurons occurs at tiny gaps called synapses, where specialized parts of the two cells (i.e., the presynaptic and postsynaptic neurons) come within nanometers of one another to allow for chemical transmission. The presynaptic neuron releases a chemical (i.e., a neurotransmitter) that is received by the postsynaptic neuron’s specialized proteins called neurotransmitter receptors. The neurotransmitter molecules bind to the receptor proteins and alter postsynaptic neuronal function. Two types of neurotransmitter receptors exist—ligand-gated ion channels, which permit rapid ion flow directly across the outer cell membrane, and G-protein–coupled receptors, which set into motion chemical signaling events within the cell. Hundreds of molecules are known to act as neurotransmitters in the brain. Neuronal development and function also are affected by peptides known as neurotrophins and by steroid hormones. This article reviews the chemical nature, neuronal actions, receptor subtypes, and therapeutic roles of several transmitters, neurotrophins, and hormones. It focuses on neurotransmitters with important roles in acute and chronic alcohol effects on the brain, such as those that contribute to intoxication, tolerance, dependence, and neurotoxicity, as well as maintained alcohol drinking and addiction.
The behavioral effects of alcohol are produced through its actions on the central nervous system (CNS) and, in particular, the brain. Synaptic transmission—the process by which neurons in the CNS communicate with one another—is a particular target for alcohol actions that alter behavior. Intoxication is thought to result from changes in neuronal communication taking place while alcohol is present in the brain. Tolerance to alcohol involves cellular and molecular adaptations that begin during alcohol exposure; the adaptations develop and diversify with repeated episodes of exposure and withdrawal and are linked to the environment present during exposure. Alcohol dependence develops after several exposure/withdrawal cycles and involves neuroadaptive changes brought about by both the exposure and withdrawal processes. Neurotoxicity produced by alcohol ingestion involves a number of cellular and molecular processes, and neurotransmitters can participate in—and modulate—many of these mechanisms. The actions of alcohol on synaptic transmission also contribute to alcohol-seeking behavior, excessive drinking, and alcoholism. Thus, understanding all of these behavioral actions of alcohol requires some knowledge of neuronal signaling in the brain and, especially, the process of synaptic transmission. This article will focus on the basic processes underlying neuronal communication and review the neuronal actions of several neurotransmitters, neurotrophic factors, and hormones thought to be involved in the neural actions of alcohol. This information, although admittedly incomplete, will provide a foundation for the detailed information on alcohol actions provided in subsequent articles in this issue and in Part 2.
Neuron-to-Neuron Communication
Neurons are the cells within the brain that are responsible for rapid communication of information. Although similar to other cells in the body, neurons are specialized in ways that set them apart from other cells and endow them with the properties that allow them to carry out their unique role in the nervous system. The neuron’s shape is one such unique feature. In addition to the cell body, or soma, which is much like that of other cells, neurons have specialized thin branches know as dendrites and axons. Neurons receive chemical input from other neurons through dendrites and communicate information to other cells through axons. Neurons also are “excitable” cells. The neuronal surface membrane contains an abundance of proteins known as ion channels that allow small charged atoms to pass through from one side of the membrane to the other. Some of these channels are opened when the voltage across the cell membrane changes. One subtype of these “voltage-gated” channels allows the neuron to produce a rapid signal known as the “action potential,” which is the fastest form of intracellular electrical signal conduction in biology (see figure 1 ).

Schematic drawing of a neuron showing dendrites, where neurons receive chemical input from other neurons; soma (cell body); and axon terminal, where neurons communicate information to other cells. Voltage-gated sodium channels in the membrane of the soma, axon, and axon terminal allow positively charged sodium ions to enter the neuron and produce rapid (in milliseconds) conduction of the excitatory action potential to the terminal. This signal stimulates neurotransmitter release at the axon terminal.
Individual neurons usually are completely separated from one another by their outer cell membranes and thus cannot directly share electrical or chemical signals. The exception to this situation is the so-called electrical synapse, in which ion-conducting pores made from proteins called connexins connect the intracellular compartments of adjacent neurons, allowing direct ion flow from cell to cell ( Kandel et al. 2000 ). This form of interneuronal communication is much less common in the mammalian CNS than chemical transmission and will not be discussed any further. Rather, the focus will be on chemical interneuronal communication involving the release of a neurotransmitter from one neuron, which alters the activity of the receiving neuron. This chemical communication usually occurs at a specialized structure called a synapse, where parts of the two cells are brought within 20 to 50 nanometers of one another (see figure 2 ). The neuron that releases the chemical is called the presynaptic neuron. A specialized structure at the tip of the axon of the presynaptic neuron, termed the axon terminal, contains small packets known as vesicles, which are filled with neurotransmitter molecules. When an action potential reaches the axon terminal and stimulates a rise in the concentration of calcium, this ion stimulates the vesicle to fuse with the cell membrane and release the neurotransmitter into the small synaptic gap between cells.

Schematic drawing of a synapse between two neurons. Synaptic vesicles contain a neurotransmitter (NT) and release it when their membranes fuse with the outer cell membrane. Neurotransmitter molecules cross the synaptic cleft and bind to receptors known as ligand-gated ion channels (LGICs) and G-protein–coupled receptors (GPCRs) on the postsynaptic neuron. GPCRs on the presynaptic neuron’s axon terminal alter the function of voltage-gated ion channels and modulate neurotransmitter release. Neurotransmitter transporters remove neurotransmitter molecules from the synaptic cleft so that they can be repackaged into vesicles.
The neuron that is acted upon by the chemical is termed the postsynaptic neuron. The neurotransmitter molecules released from the presynaptic vesicles traverse the synaptic gap and bind to proteins, termed neurotransmitter receptors, on the surface membrane of the postsynaptic neuron.
Neurotransmitter Receptors
Neurotransmitter receptors are divided into two major classes: ligand-gated ion channel (LGIC) receptors and G-protein–coupled receptors (GPCRs). LGIC receptors are proteins specialized for rapid transduction of the neurotransmitter chemical signal directly into an electrical response ( Brunton et al. 2005 ; Kandel et al. 2000 ) (see figure 3A ). One part of the protein is specialized to bind the neurotransmitter molecule. This “binding site” is on the extracellular side of the protein. The part of the protein that is buried within the cell surface membrane forms an ion pore, which is basically a fluid-filled hole in the membrane through which the charged ions can pass (ions cannot pass through lipids or other solid membrane constituents). The time between neurotransmitter binding and opening of the ion pore is on the order of microseconds to milliseconds. Thus, at synapses using ligand-gated channels, the time between action potential depolarization 1 of the axon terminal and the beginning of the current flowing through the postsynaptic LGIC is a matter of 1 to 2 milliseconds. This type of synaptic transmission produces a rapid and strong influence on postsynaptic neuron function.

Schematic drawing of a ligand-gated ion channel (left) showing the confluence of individual subunit proteins that define a pore where the ions flow across the cell membrane. A neurotransmitter binds to part of the protein located outside of the cell. Schematic drawing of a G-protein– coupled receptor (right). Neurotransmitter binds either to sites outside the cell or in a “pocket” formed by protein domains that span the membrane. The G-protein that consists of three separate protein subunits (α, β and γ, light blue) is associated with part of the protein inside the cell.
Synaptic responses mediated by LGICs are designated as excitatory or inhibitory, depending on whether their net effect is to make it more or less likely that the postsynaptic neuron will fire an action potential ( Kandel et al. 2000 ). In general, strong excitatory synaptic transmission is mediated by LGICs containing an ion pore that allows positively charged ions (i.e., cations) to flow across the membrane. Activation of such a receptor will mainly result in an influx of sodium into the cell, causing the membrane potential to depolarize, bringing it nearer to the action potential threshold. Fast inhibitory synaptic transmission usually is mediated by receptors with channels permeable to negatively charged ions (i.e., anions, usually chloride). When activated, these receptors hyperpolarize the membrane potential and/or fix the membrane potential at voltages below the action potential threshold. The types of neurotransmitters and receptors that subserve these fast excitatory and inhibitory synaptic roles are reviewed below.
GPCRs (shown in figure 3A ) are proteins that are specialized for binding the neurotransmitter molecule and subsequently producing intracellular biochemical reactions that can influence a variety of cellular functions ( Brunton et al. 2005 ; Kandel et al. 2000 ). The GPCR proteins bind directly to small intracellular proteins, known as G-proteins, as their name implies. The G-proteins are so named because they can bind the nucleotides GTP and GDP. When a neurotransmitter binds to the receptor, the exchange of GTP for GDP at the intracellular side of the protein is accelerated, and this causes the G-protein to separate into two parts (the α- and β/γ-subunits), which dissociate from the receptor protein. The liberated α- and β/γ-subunits then are free to interact with different proteins inside the cell. Both types of subunits can act on “effector” proteins to alter cellular biochemistry, physiology, and gene expression. For example, the αS-subunit binds to and activates an enzyme protein called adenylyl cyclase. The function of adenylyl cyclase is to convert the molecule adenosine triphosphate (ATP) to cyclic adenosine monophosphate (cAMP), and the resultant cAMP can act on enzymes that alter the function of cellular proteins through a mechanism called phosphorylation. In another example, free β/γ-subunits can directly bind to and activate a type of ion channel that selectively allows potassium flux across the neuronal membrane (the G-protein–activated inwardly rectifying potassium [GIRK] channel). Activation of this Gβ/γ-GIRK pathway will produce neuronal inhibition, although on a slower timescale than that produced by inhibitory LGICs. In general, the responses produced by GPCRs are slower in onset and longer lasting than those produced by LGICs because several molecular steps are required to get from the receptor protein to the final effector (in contrast to the direct intraprotein signaling within LGICs). The effects of GPCRs on neuronal physiology also often are more subtle than those produced by LGICs because they usually do not directly activate ion current in neurons. Thus, synaptic transmission mediated by GPCRs often is termed neuromodulatory.
Neurotransmitter Removal
Neurotransmitters are rapidly removed from the synapse after their release. This minimizes the time that they interact with receptors so that short, discrete synaptic signals are produced. At most synapses in the brain, specific proteins known as neurotransmitter transporters mediate this removal ( Brunton et al. 2005 ; Kandel et al. 2000 ). The transporter proteins reside in the cell surface membrane and actively move the neurotransmitter molecule from the outside to the inside of the cell (see figure 2 ). In many cases, this uptake occurs at the presynaptic terminal itself, where the neurotransmitter is directly reloaded into vesicles. However, in some cases nonneuronal support cells (i.e., glial cells) also participate in neurotransmitter uptake. Removal of synaptic neurotransmitters also can occur via enzymes that degrade the neurotransmitter to constituent molecules that do not themselves activate receptors.
Neurotrophins
In addition to neurotransmitters that alter neuronal physiology, intracellular signaling, and gene expression on a relatively fast time scale, certain small chains of amino acids (i.e., peptides) can be secreted by neurons that act as so-called growth factors or neurotrophins. The most widely known neurotrophins are nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF) and related members of this “cysteine knot” dimeric neurotrophin family that also includes neurotrophin (NT)-3 and NT-4 ( Barde 1994 ; Barde et al. 1982 ; Levi-Montalcini and Cohen 1960 ; Reichardt 2006 ). Other neurotrophins, such as glial-derived neurotrophic factor (GDNF), which acts through the transforming growth factor (TGF)β‚ signaling pathway, are peptides of a different structural class ( Unsicker 1996 ). The neurotrophins are peptides, so their primary amino acid sequence is genetically coded and is subject to alterations in the synthesis of genetic information (i.e., transcription) that can produce different variants of the mature peptide. These peptides also are generated from larger propeptides, and, thus, variations in sequence can occur at the level of posttranslational peptide processing. All of these factors combine to produce a rich variety of neurotrophins in the brain.
Neurotrophins are thought to be secreted from different neuronal structures, including both axon terminals and dendrites (see Altar and DiStefano 1998 for review). Thus, they participate in both “anterograde” signaling from the axon terminal of the presynaptic neuron to the postsynaptic elements of a downstream neuron, as well as “retrograde” signaling, in which release from dendritic elements of the postsynaptic neuron activates receptors on the presynaptic axon terminals.
Receptors for neurotrophins couple to a wide variety of intracellular signaling cascades. The main receptors for the cysteine knot family of neurotrophins are the Trk receptors ( Kaplan et al. 1991 ; reviewed by Chao and Hempstead 1995 ) (see figure 3B ). Each individual neurotrophin binds with highest affinity to a particular Trk receptor (e.g., NGF with TrkA, BDNF with TrkB), but there also are lower affinity interactions that are not as specific ( Barbacid 1995 ; Ip et al. 1993 a , b ). Upon neurotrophin binding, the Trk receptors are activated, setting into motion a variety of signaling mechanisms, including the activation of small G-proteins; activation of multifunctional protein kinases, including extracellular signal–regulated kinase (ERK) and the Fyn and Src kinases; activation of lipid-based signaling pathways; and activation of transcription factors that regulate gene expression ( Davis 2008 ). Some of these signaling pathways have effects locally within a particular subcellular compartment. Other signals (e.g., those involving transcription factors) are transmitted to the nucleus. There is evidence that neurotrophin-bound Trk receptors are internalized and translocated to the nucleus, where they can participate in signaling that regulates gene expression (reviewed by Ginty and Segal 2002 ). Internalization of neurotrophin-bound receptors also is believed to be a major mechanism by which the neurotrophins are removed from the extracellular space and ultimately degraded by intracellular peptidases. The diversity of signaling pathways activated by Trk receptors allows them to participate in a variety of neuronal functions, including not only cell survival and growth but also synaptic plasticity.

Neurotrophin binding to TRK receptors attracts a variety of intracellular signaling proteins to the intracellular portion of the TrK protein. Activation of these signaling proteins in turn activates transcription factor proteins that act on the nucleus to alter gene expression, as well as other intracellular signaling pathways that promote the growth and differentiation of neurons. Activation of neurotrophin–TrK–intracellular signaling pathways also promotes long-lasting plasticity of synaptic transmission.
BDNF = brain-derived neurotrophic factor.
Neurotrophins are well known for their ability to support the survival and growth of neurons. For example, the pioneering work of Levi-Montalcini and Cohen (1960) showed that the viability of sympathetic peripheral neurons (those outside the CNS) in culture requires NGF and that this neurotrophin stimulates outgrowth of axons and dendrites ( Levi-Montalcini 1987 ). Neurotrophins are widely expressed within the CNS. For example, BDNF is expressed in a number of brain regions, including many that have been implicated in neural mechanisms of addiction (reviewed in Davis 2008 ).
Steroid Hormones
Steroid hormones––small, complex molecules involved in intercellular communication—are highly lipid soluble and have a variety of actions in the body and brain. For example, corticosteroids are released from the cortex of the adrenal glands located on top of the kidneys in response to external stress and are carried by the bloodstream to their sites of action throughout the body and brain ( Brunton et al. 2005 ). It now is widely appreciated that steroids have two mechanisms of action. The traditional steroid signaling pathway involves an intracellular steroid receptor that resides in the cytosol when unbound and translocates to the cell nucleus when it is bound with the steroid ( Brunton et al. 2005 ; Hayashi et al. 2004 ) (see figure 3C ). The receptor protein then can bind to DNA and directly influence the transcription of a variety of genes. The second type of steroid hormone signaling involves actions on cell surface receptors. For example, derivatives of the sex steroid progesterone interact with the A-type receptors for the neurotransmitter γ-aminobutyric acid (GABA) (i.e., GABA A receptors) to enhance receptor function allosterically by producing a conformation, or shape, change in the receptor ( Mitchell et al. 2008 ) (see figure 4 ). The so-called neurosteroids that act within the brain can be generated locally within CNS tissue and thus can have paracrine 2 as well as endocrine actions.

Steroid hormones such as glucocorticoids bind to proteins within the cytoplasm of the cell. Upon steroid binding the protein moves to the nucleus where it can affect protein synthesis at the transcription step.
GR = Glucocorticoid receptor; GRE = glucocorticoid response element, a stretch of DNA that binds the GR and activates gene transcription.

Schematic drawing of the γ-aminobutyric acid receptor (GABA A ) ligand-gated ion channel complex. The receptor molecule is formed by the confluence of five subunit proteins. In this case, two of the subunits are of the α type, two β and one γ, although many combinations of the 20 known subunits are possible. Globular regions of the protein stick out from the membrane on the extracellular side, and the interfaces between these regions are targets for GABA and for the benzodiazepines and related drugs. The protein domains that span the outer cell membrane are depicted as cylinders. These regions are thought to be targets for general anesthetics (e.g., propofol) neurosteroids, and alcohol. A hole in the middle of the five subunits is the ion conduction pathway, or channel pore.
Receptor Pharmacology
Before discussing specific neurotransmitter molecules and their cognate receptors, it is important to review terminology related to receptor action (see also Brunton et al. 2005 ). The term agonist refers to a molecule that binds to and activates the receptor. Of course, the neurotransmitter itself is the natural receptor agonist. However, chemists have been able to purify or synthesize other small molecules that mimic the effect of the neurotransmitter. Other small molecules, called competitive antagonists, can bind to the site normally occupied by the neurotransmitter and prevent receptor activation. These competitive antagonist compounds do not activate the receptor but prevent the neurotransmitter from binding. Antagonists also may change the conformation of the receptor such that activation is more difficult. The term competitive stems from the fact that the neurotransmitter (or agonist) and the antagonist “compete” for binding to the same part of the receptor, and increasing the concentration of one molecule can overcome the effects of the other. Other types of antagonists bind to parts of the receptor protein that are distinct from the agonist binding site. For example, noncompetitive antagonists react with the receptor and prevent activation in an allosteric manner even when the neurotransmitter molecule binds to the protein. In this case, increasing the concentration of neurotransmitter or agonist cannot overcome antagonist actions, and there is no “competition” for the binding site. Other naturally occurring and synthetic molecules enhance receptor function by binding to a region of the receptor distinct from the neurotransmitter/agonist binding site and improving the efficiency of receptor activation. These compounds generally are known as allosteric enhancers of receptor function.
Receptor agonists, antagonists, and allosteric modulators are used as pharmaceutical treatments for a variety of neurological and psychiatric disorders ( Brunton et al. 2005 ). For example, a small molecule called baclofen can control certain types of movement spasticity through its agonist action at the B-type receptor for the neurotransmitter GABA ( Bowery 2006 ). Many of the major antipsychotic drugs used in schizophrenia treatment, such as Haldol ® , are competitive antagonists at the type 2 receptor for the neurotransmitter dopamine ( Kapur et al. 2006 ). In addition, Valium ® (also known as diazepam), Ambien ® (also known as zolpidem), and related antianxiety and sleep aid drugs are allosteric enhancers of the GABA A receptor ( Sanger 2004 ), which is the other major receptor for this neurotransmitter. Indeed, neurotransmitter receptors are the predominant targets for therapies aimed at treatment of brain disorders.
The Neurotransmitter Molecules
Many small organic molecules serve as neurotransmitters in the brain. For example, amino acids such as glutamate and glycine, which are well known as constituents of proteins, also act as neurotransmitters ( Kandel et al. 2000 ). Histamine, a molecule that has a prominent role in inflammation and infection in the body, also is a neurotransmitter ( Haas and Panula 2003 ). A variety of peptides also have been found to act as neurotransmitters ( Kandel et al. 2000 ). A review of all neurotransmitters is beyond the scope of this article. Rather, the sections that follow focus on those neurotransmitters whose actions are most strongly implicated in alcohol intoxication, tolerance, dependence, and addiction. This discussion is organized according to the neurotransmitters’ proposed roles within the chronology of alcohol actions. Those neurotransmitters thought to be most heavily involved in intoxication are discussed first, followed by those involved in chronic alcohol effects. The final section addresses those neurotransmitters that do not appear to be direct targets for the neural actions of alcohol but which may be involved in alcohol abuse and addiction and are potential pharmacotherapeutic targets.
GABA mediates the majority of fast synaptic inhibition in the brain, specifically through activation of GABA A receptors. Like glutamate, GABA also is found in all brain regions. The intrinsic ion channel contained in the GABA A receptor protein is permeable to Cl − and other anions ( Kandel et al. 2000 ). Activation of the receptor can hyperpolarize neurons through the influx of negative charges at membrane potentials below the threshold for action potential generation. This inhibition generally counteracts the effect of glutamate and other depolarizing, excitatory synaptic influences. The amino acid glycine produces a similar action in the spinal cord and posterior parts of the brain.
Several subtypes of GABA A receptors exist; they are formed by the confluence of individual “subunit” proteins (see figure 4 ). Each subunit has a slightly different amino acid sequence, and there are 20 subunits in all in the mammalian brain ( Sieghart and Sperk 2002 ). Thus, a variety of different types of GABA A receptors are made in different brain neurons. GABA A receptors are present both at the synapse and on postsynaptic membranes distant from synapses. These latter “extrasynaptic” GABA A receptors are sensitive to very low levels of extracellular GABA, and thus they often produce a continuous inhibitory tone that helps to set the resting potential of certain neurons ( Stell et al. 2003 ).
The GABA A receptor channel contains numerous sites for allosteric modification. Perhaps the best known among these is the “benzodiazepine” binding site ( Sanger 2004 ; Sigel 2002 ). This site resides on the extracellularly exposed part of the protein at a site distinct from the agonist binding region. Many compounds, such as Valium, that bind to this site have potent allosteric enhancing effects on the receptor. Other compounds can have an antagonist action at this site and will prevent allosteric enhancement by benzodiazepines without altering the response of the receptor to GABA ( Mohler 1983 ). The diversity of GABA A receptor subtypes has given rise to different benzodiazepine effects on different receptors and different brain neurons, providing multiple possibilities for pharmaceutical development.
Drugs that target the GABA A receptor have been in widespread use for treatment of disorders ranging from anxiety to epilepsy ( Mohler 2006 ; Sigel 2002 ). The majority of general anesthetics currently in use produce their actions predominantly through enhancing GABA A receptor function ( Hemmings et al. 2005 ). Almost all of these therapeutic drugs either directly activate the GABA A receptor or activate the benzodiazepine site to produce allosteric enhancement of the receptor, as mentioned above.
GABA also can act through a GPCR, the aforementioned GABA B receptor. This receptor acts to inhibit neuronal activity in two ways ( Bowery et al. 1990 ). First, activation of GABA B receptors leads to a direct G-protein–mediated activation of the GIRK-type potassium channel. This channel can hyperpolarize neurons and counteract synaptic excitation. This type of GABA B -mediated modulation is found in many postsynaptic neuronal elements throughout the brain. GABA B receptors also are found on presynaptic terminals of neurons in many brain regions ( Bowery et al. 1990 ). When activated, these receptors inhibit neurotransmitter release via mechanisms that involve inhibition of voltage-gated calcium channels whose function is necessary for proper release. Presynaptic GABA B receptors are found on the axon terminals of both GABAergic and glutamatergic neurons, and thus the net effect of receptor activation can be either disinhibitory or inhibitory depending on which receptors are activated.
GABAergic transmission is a sensitive target for both the acute and chronic effects of alcohol (reviewed in Lovinger and Homanics 2007 ; Siggins et al. 2005 ). Acute alcohol can produce allosteric enhancement of the function of GABA A receptors, although this effect is not observed at all GABA A receptors, and there is some debate as to which receptor subtypes are most sensitive to alcohol (reviewed in Lovinger and Homanics 2007 ). Researchers have observed effects at concentrations as low as the low millimolar range (the levels reached with ingestion of a single drink), suggesting that even some aspects of low-dose intoxication might involve enhanced GABA A receptor function. Acute ethanol also enhances the release of GABA at a number of synapses in the brain ( Siggins et al. 2005 ). The mechanisms of this presynaptic enhancement are just beginning to be explored. One interesting set of studies indicates that activation of presynaptic GABA B receptors prevents alcohol potentiation of GABA release and can bring about a “tolerance” to the alcohol action ( Ariwodola and Weiner 2004 ). Potentiation of GABAergic synaptic transmission appears to contribute to a number of aspects of acute alcohol intoxication, including motor incoordination, anxiety-reducing effects, sedation, and the internal cues that signal intoxication ( Lovinger and Homanics 2007 ; Siggins et al. 2005 ; Vengeliene et al. 2008 ).
The brain’s GABAergic system also shows marked changes following chronic alcohol exposure. Some of these changes are likely to be adaptations to the acute alcohol actions that potentiate GABAergic transmission. The best-characterized changes involve alterations in the subunits that make up the GABA A receptor ( Kumar et al. 2004 ), which alter the efficacy and timing of inhibitory synaptic transmission. Research also shows that chronic alcohol exposure can produce decreases and increases in GABA release in different brain regions (reviewed in Weiner and Valenzuela 2006 ). The predominant effect of these chronic alcohol effects is to make the brain hyperexcitable during withdrawal from chronic alcohol exposure. This can produce effects such as heightened anxiety and even overt seizures during withdrawal ( Krystal et al. 2006 ; Kumar et al. 2004 ). Benzodiazepines commonly are used to treat alcohol withdrawal because of their effectiveness in controlling these aspects of hyperexcitability ( Krystal et al. 2006 ). Drugs that target GABAergic transmission also have been touted as potential pharmacotherapeutic treatments for alcohol abuse and alcoholism ( Koob 2004 ; Krystal et al. 2006 ), but, as yet, no drugs that explicitly and specifically target GABAergic mechanisms are in clinical use for this purpose.
Glutamate is the major excitatory neurotransmitter in the mammalian brain. Fast transmission mediated by this neurotransmitter accounts for synaptic excitation of most, if not all, brain neurons ( Kandel et al. 2000 ), and thus glutamate is found throughout the brain. It is not surprising, therefore, that glutamate has key roles in a wide variety of brain functions. The fast synaptic excitation produced by glutamate involves the activation of three major subtypes of LGICs, termed the AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) 3 -, kainate-, and NMDA ( N -methyl- d -aspartic acid) 4 -type receptors based on the synthetic agonists that best activate each receptor ( Kandel et al. 2000 ). At most excitatory synapses, glutamate released from presynaptic vesicles crosses the synapse and binds to AMPA-type glutamate receptors. The binding of glutamate to AMPA receptors directly activates the ion pore intrinsic to this protein and produces a rapid depolarization of the postsynaptic neuron that increases the likelihood that this neuron will fire an action potential. Thus, timely and accurate communication within brain circuits depends to a large extent on proper AMPA receptor activation.
The function of NMDA-type glutamate receptors is more complicated. When glutamate binds to the NMDA receptor, activation of the intrinsic ion pore is favored, as is the case for AMPA receptors and other LGICs. However, at membrane potentials near the resting potential (e.g., −60 to −70 mV), the ion pore of the NMDA receptor is occluded by magnesium ions ( Kandel et al. 2000 ). Magnesium is present at millimolar concentrations in the extracellular solution and binds to a site within the NMDA receptor ion pore that becomes accessible very shortly after the ion pore opens. This magnesium pore blocking action is stronger at more negative membrane potentials and is thus reduced as the membrane potential becomes more depolarized. In practice, at a glutamatergic synapse that contains both AMPA and NMDA receptors, the initial action of glutamate will be to activate AMPA receptors. This will depolarize the cell because of the influx of cations through the AMPA receptor ion pore. If the depolarization is sufficiently strong and glutamate still is present, the NMDA receptor pore will become unblocked, and ion flux through this receptor-channel also can participate in activating the postsynaptic neuron.
The AMPA and NMDA receptors have central roles in producing long-lasting changes in synaptic function that are thought to represent molecular mechanisms of learning and memory ( Kandel et al. 2000 ). One such change, named long-term potentiation, is a long-lasting increase in the strength of signaling at glutamatergic synapses that mediate fast excitatory synaptic transmission. When these synapses are activated repeatedly and perseveratively (e.g., by high-frequency synaptic input) the repeated activation of AMPA receptors will depolarize the postsynaptic neuron sufficiently to allow for ion current to flow through NMDA receptors. The ion pore of the NMDA receptor is especially permeable to calcium ions that can activate a variety of intracellular signaling cascades, including protein kinases. Strong NMDA receptor activation sets into motion a sequence of molecular events that result in the insertion of additional AMPA receptors at the synapses, thus strengthening synaptic transmission ( Kandel et al. 2000 ). There now is abundant evidence that this NMDA receptor–dependent long-term potentiation is involved in a variety of types of learning in the brain ( Robbins and Murphy 2006 ).
Glutamate also can activate GPCRs known as metabotropic glutamate receptors (mGluRs) ( Kandel et al. 2000 ). Eight mGluRs are known to exist and can be separated into three groups termed I, II, and III ( Coutinho and Knopfel 2002 ). The group I mGluRs couple through the G q -type G-protein subtype to activate enzymes called phospholipases. These receptors mainly are on postsynaptic structures, where they serve to activate intracellular signaling that modifies ion channel function and biochemical processes including the generation of second messengers 5 from cell membrane lipids. The group II and III mGluRs couple to G i/o -type G-proteins. Members of these latter two mGluR subgroups reside predominantly on presynaptic axon terminals, where they act to inhibit neurotransmitter release ( Coutinho and Knopfel 2002 ).
Therapeutic uses for glutamate receptor–targeted drugs are a matter of intensive research at present. Ketamine is an antagonist of NMDA receptor function that has been used as a general anesthetic in animals and, in some cases, humans ( Sinner and Graf 2008 ). Recent studies suggest that group II mGluR agonists may be useful in the treatment of psychosis ( Patil et al. 2007 ).
Acute alcohol exposure generally inhibits glutamatergic synaptic transmission ( Siggins et al. 2005 ; Woodward 2000 ). At most synapses in the CNS, NMDA receptor function is the aspect of glutamatergic synaptic transmission that is most sensitive to alcohol inhibition ( Woodward 2000 ). Alcohol also inhibits synaptic plasticity that requires NMDA receptor activation, and this effect may contribute to the memory-impairing effects of alcohol. Alcohol interactions with NMDA receptors also may contribute to its intoxicating effects ( Krystal et al. 2003 ).
Chronic alcohol exposure results in an increase in NMDA receptor number and function that likely is a compensatory change induced by the inhibitory effect of acute alcohol ( Fadda and Rossetti 1998 ). This increased NMDA receptor function is thought to contribute to withdrawal hyperexcitability and perhaps also to alcohol-induced neuronal damage. A hyperglutamatergic state also may result from chronic alcohol exposure, and there is growing evidence that decreasing this excessive glutamatergic transmission might be helpful in reducing relapse to alcohol drinking ( Vengeliene et al. 2008 ). Indeed, a few drugs that target glutamatergic transmission, either directly or indirectly, have been used for treatment of alcoholics or currently are being tested for their efficacy in reducing drinking and/or relapse in alcoholics ( Krystal et al. 2003 ).
The neurotransmitter dopamine has been widely implicated in a variety of neuronal functions, including brain mechanisms of reward, evaluation of environmental stimuli, general behavioral activity levels, and disorders such as Parkinson’s disease and schizophrenia ( Brunton et al. 2005 ; Kandel et al. 2000 ). Considering that dopamine is made by very few cells in the brain and acts mainly within a subset of brain regions (see figure 5A ), this neurotransmitter seems to have a disproportionately large impact on brain function.

Neurotransmitters with discrete localization within the brain. A) The chemical structure of the monoamine neurotransmitter dopamine and a schematic drawing of the localization of dopamine-containing neurons in the human and rat brain and the sites where dopamine-containing axons are found. B) The chemical structure of the monoamine neurotransmitter serotonin and similar brain map showing locations of serotonin-containing cells and their axons.
Dopamine acts exclusively via activation of GPCRs and thus is a pure neuromodulator. There are five identified dopamine receptors that fall into two subclasses: those that activate G s /G olf -type G-proteins (D1 and D5) and those that activate G i/o -type G-proteins (D2-4) ( Lachowicz and Sibley 1997 ). In general, the two classes of receptors produce separate, and often opposing, effects on neuronal physiology. For example, D1-like receptors enhance sodium channels in neurons from the brain region known as the striatum, whereas D2 receptors inhibit these same channels ( Surmeier and Kitai 1993 ). However, there are situations in which the two dopamine receptor subtypes appear to work together or even produce synergistic actions ( Lee et al. 2004 ). Within the striatum, D1 and D2 receptors mainly are segregated onto two different types of neurons ( Gerfen 1992 ). The medium spiny neurons provide the only source of output from this brain region. Half of these neurons connect to a brain region called the substantia nigra pars reticulata, forming the so-called “direct” pathway that tends to activate the cortex. The other half connect to a brain area called the globus pallidus internal segment and thus form the “indirect” pathway that tends to dampen cortical output. The coordination of these two pathways determines one’s ability to initiate actions and control action sequences. Thus, dopamine, working through these two receptor subtypes, has key roles in controlling performance of actions, including those affected by intoxication and those involved in addiction.
Dopaminergic transmission is targeted by many drugs, including both legal prescription medications and illegal drugs of abuse. Perhaps the most widely known brain disorder involving the brain dopaminergic system is Parkinson’s disease. This debilitating and ultimately lethal neurological disorder arises from the death of dopaminergic neurons and the resultant loss of brain dopamine ( Kandel et al. 2000 ). The most common therapy for this disease involves dopamine replacement by treatment with the dopamine precursor l -Dopa ( Stacy and Galbreath 2008 ), which is taken up by the brain and used to make more dopamine. Dopamine receptor agonists also are sometimes used as an adjunct therapy with l -Dopa ( Stacy and Galbreath 2008 ). Agonists of the D 2 class of dopamine receptors are used in the treatment of the movement disorder known as restless legs syndrome ( Winkelman et al. 2007 ). Dopamine receptor- and transporter-targeted drugs also have a large role in the treatment of other neurological and neuropsychiatric disorders. The majority of antipsychotic drugs have potent actions at the D 2 -like dopamine receptors, although they certainly have effects on other targets that could contribute to their therapeutic efficacy ( Kapur et al. 2006 ). Drugs that block the dopamine transporter, such as methylphenidate (Ritalin ® ) are used in the treatment of attention deficit hyperactivity disorder (ADHD) ( Arnsten 2006 ) (note the potential abuse liability of this class of drugs, as discussed below) ( Faraone and Wilens 2007 ). Antagonists for D 2 dopamine receptors also are used clinically to reduce vomiting in circumstances such as chemotherapy ( Reddymasu et al. 2007 ).
Cocaine, amphetamine, and other “stimulant” drugs block or reverse the action of the dopamine transporter ( Amara and Sonders 1998 ). The net effect of these drugs is to increase levels of dopamine within the synapse. Although these drugs act on other molecular targets, the evidence is fairly convincing that it is the effect on dopaminergic transmission that accounts for the majority of the intoxicating and addictive actions of these drugs. Most other drugs of abuse also influence the brain dopaminergic system. Nicotine stimulates the activity of the dopaminergic neurons themselves ( Pidoplichko et al. 1997 ) and also can activate dopamine release from axon terminals ( Grady et al. 2007 ). Morphine and other opiate drugs depress the activity of GABAergic interneurons and, through this effect, indirectly increase the activity of dopaminergic neurons ( Johnson and North 1992 ). Alcohol also increases the activity of these neurons, likely via both direct actions on the neurons and indirect actions through other neurons ( Brodie et al. 1999 ; Ericson et al. 2008 ; Gessa et al. 1985 ; Okamoto et al. 2006 ).
Dopaminergic transmission has been implicated in the actions of almost all drugs of abuse, and transmission mediated by this neurotransmitter is altered by alcohol exposure in both the acute and chronic phases (reviewed in Vengeliene et al. 2008 ). The acute alcohol-induced increase in firing of dopaminergic neurons appears to drive increases in extracellular dopamine levels in the brain regions to which these neurons project (reviewed in Gonzales et al. 2004 ). Imaging of dopamine receptors in the human brain also suggests that acute alcohol exposure alters dopamine levels in key brain structures (reviewed in Wong et al. 2003 ). These effects may contribute to the process by which animals encode the reinforcing value of alcohol.
As animals learn to consume alcohol in the laboratory, increases in brain dopamine levels become associated with stimuli that predict access to alcohol ( Gonzales et al. 2004 ). Thus, dopamine also plays a role in learning about environmental contexts that encourage drinking. Chronic alcohol consumption can lead to a hypodopaminergic state that motivates the drinker to seek alcohol in order to restore the desired levels of the neurotransmitter ( Volkow et al. 2007 ). However, despite these findings, pharmacotherapies aimed at the dopaminergic system have not shown particular efficacy in reducing alcohol drinking either in animal models or in humans with alcohol abuse disorders. Perhaps the development of drugs with greater specificity for certain subtypes of dopamine receptors will prove more efficacious in this context.
Adenosine is a purine nucleoside (a compound with a nitrogen-containing base linked to a sugar molecule) that is produced during nucleic acid metabolism. This compound also participates in a number of types of cell–cell communication, including synaptic transmission in all regions of the nervous system. Adenosine primarily is a neuromodulatory transmitter and produces its actions via the activation of two main types of GPCRS, the A1 and A2a adenosine receptors ( Fredholm et al. 2005 ). Other adenosine receptors exist but are present only in small quantities within the CNS.
Activation of A1 adenosine receptors in turn activates G i/o class G-proteins. In general, these G-proteins activate GIRK potassium channels, inhibit voltage-gated calcium channels, inhibit adenylyl cyclase, or activate phosphorylation of certain intracellular protein kinase enzymes ( Fredholm et al. 2005 ). Adenosine A2a receptors activate G s or G olf –type G-proteins ( Fredholm et al. 2007 ). These G-proteins stimulate adenylyl cyclase to enhance production of the second messenger cyclic AMP ( Fredholm et al. 2007 ).
After producing its actions on synaptic adenosine receptors, the neurotransmitter is transported back into cells via a cell membrane neurotransmitter transporter protein known as the adenosine transporter ( Fredholm et al. 2005 ). Adenosine also can be metabolized by enzyme proteins known as adenosine deaminase and adenosine kinase ( Fredholm et al. 2005 ). The transporter and enzymes regulate the duration of adenosine signals within the synaptic cleft. Inhibition of either process can prolong the time that the neurotransmitter is present in the synapse and, consequently, the duration of activation of the adenosine receptors.
The behavioral effects of modifying brain adenosinergic communication are very familiar to many of us. Caffeine acts as an adenosine receptor antagonist ( Dunwiddie and Masino 2001 ). The major behavioral effects of caffeine, including enhanced activity and focused attention, appear to involve inhibition of the function of both A1- and A2a-type adenosine receptors, although locomotor stimulation by caffeine is predominantly because of A2a antagonism.
Acute alcohol exposure increases adenosine signaling in cell lines of neural origin ( Nagy et al. 1990 ). This effect appears to involve inhibition of a nucleoside transporter that normally produces rapid uptake of adenosine into cells. This inhibitory action increases extracellular adenosine levels and prolongs the duration of adenosine signaling to the cell. The role of these changes in adenosinergic transmission in acute intoxication is not clear, although removing the alcohol-sensitive nucleoside transporter in mice alters intoxication and alcohol intake ( Choi et al. 2004 ). Chronic alcohol exposure causes a compensatory downregulation of A2a adenosine receptors and decreases in the adenylyl cyclase signaling by these receptors ( Diamond et al. 1991 ). These receptors predominantly are found in brain regions involved in neural processes important for reward, habit formation, and addiction ( Jarvis and Williams 1989 ). Thus, alterations in adenosine signaling in these brain regions could contribute to alcohol addiction ( Choi et al. 2004 ; Naassila et al. 2002 ).
The neurotransmitter serotonin (also known as 5-hydroxytryptamine or 5-HT) is a close molecular relative of dopamine that belongs to the family of neurotransmitters known as monoamines ( Kandel et al. 2000 ). Like dopamine, serotonin is made by small discrete clusters of neurons located at the base of the brain (see figure 5B ). These serotonergic neurons connect to other neurons located throughout the CNS, including neurons in the cerebral cortex and other forebrain structures. Thus, serotonin has the capacity to influence a variety of brain functions including sensations related to environmental stimuli, pain perception, learning and memory, and sleep and mood ( Kandel et al. 2000 ). The role of serotonin in mood control has received considerable attention in both the laboratory and the clinic, as selective serotonin reuptake inhibitors (SSRIs) such as Prozac ® are the most widely prescribed drugs for depression and other mood disorders ( Brunton et al. 2005 ; Kandel et al. 2000 ).
The majority of serotonin actions in the brain occur via activation of GPCRs. The human brain has 15 serotonin-activated GPCRs that activate a wide variety of G-protein subtypes ( Kitson 2007 ). Thus, serotonin can produce neuromodulatory effects that tend to either increase or decrease neuronal output. Different subtypes of these GPCRs are found on presynaptic and postsynaptic neuronal structures in different brain regions. By activating these receptors, serotonin can enhance or inhibit neurotransmitter release at certain synapses and can produce slow hyperpolarizing or depolarizing synaptic responses at others. Intracellular signaling via serotonin-activated GPCRs also can influence the function of intracellular enzymes as well as alter gene expression.
Serotonin also can activate a single type of LGIC-type neurotransmitter receptor, the so-called 5-HT 3 receptor ( Thompson and Lummis 2007 ). This receptor contains a channel that is permeable to positively charged cations and thus produces fast activation of neurons when bound to serotonin. One interesting facet of the action of this receptor is that often it resides on axon terminals that contain and release GABA. Thus, activation of these presynaptic 5-HT 3 receptors leads to a rapid release of GABA that will then inhibit downstream neurons.
In addition to the use of SSRIs for neuropsychiatric therapy, as mentioned above, a variety of other therapeutic uses exist for serotonergic drugs. The SSRIs produce their actions via inhibition of the serotonin transporter protein, as their name implies. Buspirone is an anxiety-reducing drug that acts on the 5-HT-1 A receptor ( Barrett and Vanover 1993 ), and use of this drug has been suggested for other disorders. The 5-HT 3 antagonists routinely are used to reduce nausea and vomiting resulting from chemotherapy and can be used for similar purposes following surgical anesthesia, and these drugs also have been used for treatment of irritable bowel syndrome ( Thompson and Lummis 2007 ).
The brain serotonergic system also is the target of psychoactive drugs, including many that are illegal. The largest class of hallucinogenic drugs, including LSD, mescaline, and psilocybin, are all partial agonists of the 5-HT 2 receptor subtypes, and the 5-HT 2A receptor is implicated in the effects of these drugs ( Fantegrossi et al. 2008 ). Several amphetamine derivatives, such as 3,4-methylenedioxymethamphetamine (MDMA, also known as ecstasy), alter serotonin transporter molecules and increase synaptic serotonin levels ( McKenna and Peroutka 1990 ). This may account for their sensory-enhancing effects.
Acute alcohol has mixed effects on serotonergic transmission (reviewed in Lovinger 1997 ). A slowing of serotonergic reuptake is observed ( Daws et al. 2006 ), but this does not appear to be because of impairment of the serotonin transporter targeted by SSRIs. Alcohol also potentiates the function of the 5-HT 3 receptor ( Lovinger 1999 ).
Chronic alcohol exposure has been demonstrated to interact with various aspects of serotonergic transmission that could alter anxiety and affect (reviewed in Lovinger 1997 ). Based on the well-known role of serotonin in neural mechanisms underlying mood and stress responses, pharmacotherapies aimed at the serotonergic system have long been touted as potential treatments for alcoholism (reviewed in Ait-Daoud et al. 2006 ). Treatments with SSRIs are efficacious in reducing alcohol intake in laboratory animals ( LeMarquand et al. 1994 ; Pettinati et al. 1996 ) but have had mixed success in humans ( Ait-Daoud et al. 2006 ; Naranjo and Kadlec 1991 ). It is possible that these drugs might work best in patients with comorbid depression. The 5-HT 3 antagonist ondansetron reduces relapse in alcoholics, particularly in those with early onset (reviewed in Ait-Daoud et al. 2006 ).
Opioids and Other Peptides
Peptides have long been known to function as hormones within the body and to participate in synaptic communication in the brain ( Brunton et al. 2005 ; Kandel et al. 2000 ). All of the known peptide actions in the brain are neuromodulatory, and thus these agents act through GPCRs. Many peptides act as neurotransmitters (i.e., the so-called neuropeptides); this section will focus on a few neuropeptides that have been implicated in alcohol actions on the brain.
Most of the neurotransmitter molecules discussed above are synthesized within the axon terminal itself and are then delivered into the vesicles waiting near the presynaptic release sites. Peptide neurotransmitters, however, normally are synthesized in the neuronal cell body, some distance from the site of release ( Kandel et al. 2000 ). The neuropeptides then are packaged into vesicles and transported inside the vesicle to the axon terminal. Thus, it is somewhat easier to deplete the store of neuropeptide transmitters in a given axon terminal, and it can only be refilled after transport from the cell body.
The opioid peptides are some of the most widely known neuropeptides. These include β-endorphin (which consists of 31 amino acids), dynorphin (13 amino acids), and the enkephalins (5-amino-acid peptides that end in either methionine [met-enkephalin] or leucine [leu-enkephalin]) ( Kandel et al. 2000 ). The notoriety of these peptides stems from the fact that they serve as agonists for the receptors that also are activated by morphine, heroin, and the other opiate drugs. These receptors are known as opiate receptors, and there are three different subtypes designated the μ, δ, and κ receptors ( Connor and Christie 1999 ). In addition, a peptide known as nociceptin or orphanin FQ has actions at a separate opiate-like receptor called the ORL-1 receptor ( Connor and Christie 1999 ). All of these receptors preferentially couple to G i/o -type G-proteins and thus generally are known to inhibit neurotransmitter release and reduce the activity of neurons ( Williams et al. 2001 ).
Opioid peptides are found in many regions of the nervous system. Like other neuropeptides, the opioids usually are stored in and released from large vesicles different from those that contain the small molecules (e.g., glutamate or GABA). These opioid peptide–containing vesicles often are found in the same axon terminals with the smaller diameter, small molecule–containing vesicles, and thus many neurons have the capacity to release both small molecule and opioid peptide neurotransmitters ( Kandel et al. 2000 ). Opiate receptors are found on both presynaptic and postsynaptic structures ( Williams et al. 2001 ). The termination of transmission mediated by opioid peptides involves peptidases, which are specific enzymes that catalyze the degradation of the peptide into its constituent amino acids ( Schwartz et al. 1981 ). These amino acids are then taken back into cells via amino acid transporters, where they can be used for future peptide or protein synthesis.
Opiate receptors have been targeted for a number of therapeutic uses. Morphine, heroin, fentanyl, and other powerful opiate receptor agonists have long been used for pain reduction ( Brunton et al. 2005 ). The opiate receptor partial agonist methoadone is a well-known and effective treatment for addiction to opiate drugs ( Brunton et al. 2005 ), basically substituting for morphine or heroin to prevent withdrawal symptoms without having the same debilitating effects.
Of course, opiate agonists are among the drugs with the strongest abuse/addiction liability ( Brunton et al. 2005 ). Injectable heroin is perhaps the best-known addictive opiate and continues to be a problem for people worldwide. However, abuse of powerful synthetic opiates taken in pill form, such as Oxycontin ® (also known as Oxycodone), has been on the rise in recent years ( Compton and Volkow 2006 ). Another psychoactive drug, the plant derivative known as salvanorin A, acts as an agonist at κ-opioid receptors ( Roth et al. 2002 ). When ingested, it produces a relatively short-lasting disorientation, such that the user becomes unaware of his/her location in space and time.
Acute alcohol alters endogenous opioid peptides and opiate receptors ( Charness 1989 ; Gianoulakis 1989 ). However, the contribution of these actions to intoxication remains unclear. Chronic alcohol exposure also alters brain opiatergic systems ( Charness 1989 ; Gianoulakis 1989 ). Interestingly, opiate receptors have emerged as useful targets for pharmacotherapeutic treatment of alcohol use disorders (reviewed in O’Brien 2005 ; O’Malley and Froehlich 2003 ). Use of the general opiate receptor antagonist naltrexone is approved for the treatment of alcoholics. This compound also reduces alcohol drinking in rodents, apparently via blockade of the μ-type opiate receptor ( Altshuler et al. 1980 ; Gonzales and Weiss 1998 ; Krishnan-Sarin et al. 1998 ). The mechanism of action may involve a reduction of endogenous opioid peptide actions that normally promote increases in dopamine release ( Gonzales and Weiss 1998 ).
Many other neuropeptides originally found to act as hormones also have been found to act as neurotransmitters. Corticotrophin-releasing hormone (CRH) was originally known for its role in the pituitary gland, where it stimulates a cascade of molecular processes that ultimately leads to the release of the cortisone-like steroid hormones (e.g., corticosteroids) ( Brunton et al. 2005 ). Within the brain, CRH can communicate signals related to stress, mood, and changes in other bodily functions ( Reul and Holsboer 2002 ). The cellular release of this neuropeptide is stimulated by alcohol ( Nie et al. 2004 ) as well as by exposure to stressful stimuli. Mounting evidence suggests that CRH and its receptors participate in the interactions between stress and alcohol, including increased drinking or relapse to drinking following stressful events ( Heilig and Koob 2007 ).
A variety of other neuropeptides have been implicated in brain responses to alcohol and alcohol drinking behavior (see Thorsell 2007 and Wurst et al. 2007 for more information).
Endocannabinoids and Other Lipid-Derived Neuromodulators
Molecules derived from the chemical modification of the lipids found in neuronal membranes are another class of neuromodulatory substances. Throughout the body, lipid-derived molecules are known to have paracrine actions. The lipid metabolites known as prostaglandins are perhaps the best-known examples of such paracrine agents ( Brunton et al. 2005 ). It is worth noting that lipid-derived compounds do not have to be released from synaptic vesicles. Unlike other neurotransmitters, they usually escape from neurons directly through the cell surface membrane.
Many of these lipid-derived agents are involved in synaptic communication. Endocannabinoids (a contraction of endogenous cannabinoids) are an example ( Chevaleyre et al. 2006 ). Arachidonoyl ethanolamide (AEA) and 2-arachidonoyl glycerol (2-AG) are two compounds that can be produced upon breakdown of membrane lipids that contain the fatty acid known as arachidonic acid. These compounds are produced throughout the brain and body and have been implicated in a number of physiological functions. Endocannabinoids have an intriguing retrograde signaling action at synapses ( Alger 2002 ). In other words, synaptic communication by these compounds generally occurs in a direction opposite to that of traditional neurotransmitters. The endocannabinoids often are produced by postsynaptic neuronal elements and act on their cognate receptors, cannabinoid 1 (CB1) receptors, which are found almost exclusively on presynaptic axon terminals in the brain. This signaling requires that the compound traverse the synapse in a backward, or retrograde, direction. This action of endocannabinoids has now been found throughout the nervous system.
The CB1 receptor is a GPCR that links to G i/o -type G-proteins. The most common effect of activating this receptor is inhibition of neurotransmitter release ( Lovinger 2008 ). Thus, retrograde endocannabinoid signaling results in decreased release of several types of neurotransmitters, including both GABA and glutamate. Endocannabinoid effects are therefore disinhibitory (relief from GABAergic inhibition) or inhibitory (decreased glutamatergic excitation) depending on the brain region and synapses in which they act. The presence of endocannabinoids in the synapse often leads to inhibition of neurotransmitter release that persists for as long as the endocannabinoid is present ( Chevaleyre et al. 2006 ; Lovinger 2008 ). Endocannabinoids also can trigger a long-lasting depression of neurotransmitter release called long-term synaptic depression (LTD) ( Chevaleyre et al. 2006 ; Lovinger 2008 ). This depression is set into motion by endocannabinoid activation of CB1 receptors but does not require sustained CB1 activation for its long-term maintenance. Accumulated evidence suggests that the synaptic depression produced by retrograde endocannabinoid signaling has key roles in brain mechanisms of learning and memory as well as addiction ( Lovinger 2008 ).
The term “cannabinoid” within the name of these compounds reflects that fact that the endocannabinoids have something in common with drugs derived from the Cannabis sativa plant, such as marijuana. Indeed, the CB1 receptor is itself the major target for Δ-9tetrahydrocannabinol (Δ-9THC), the primary psychoactive ingredient in cannabis-derived drugs ( Brunton et al. 2005 ). The endocannabinoids produce much milder and shorter-lasting versions of the effects produced by cannabinoids in the brain, including relief from anxiety, relief from pain, actions that affect movement initiation, balance and coordination, and effects on cognition ( Lovinger 2008 ; Piomelli et al. 2000 ). Knowledge of the neuronal effects of Δ-9THC provides a great deal of information on the effects of specific CB1 agonists. Indeed, a number of synthetic agonists have been developed for this receptor, and they produce strong intoxication, movement impairment, decreased learning and short-term memory, pain relief, and, at high doses, a loss of movement known as catalepsy ( Howlett 1995 ). At low-to-moderate doses, these compounds also stimulate appetite, a well-known effect of marijuana and other illegal cannabis-derived drugs ( Di Marzo et al. 2001 ).
Antagonists for the CB1 receptor also have neural actions, and there is increasing evidence of the therapeutic usefulness of these compounds. Studies in laboratory animals indicate that CB1 antagonists reduce feeding and the metabolic changes that often accompany obesity ( Di Marzo et al. 2001 ; Ravinet Trillou et al. 2003 ). The CB1 antagonist known as Acomplia ® , Rimonabant, or SR141716 already is in use in Europe for treatment of obesity and the associated metabolic disorder. Antagonists for this receptor also reduce self-administration of a number of drugs of abuse, most notably nicotine and alcohol ( Maldonado et al. 2006 ; Wang et al. 2003 ). Indeed, researchers have observed a variety of interactions between alcohol and the brain endocannabinoid system, suggesting involvement of this system in alcohol dependence ( Colombo et al. 2007 ). Research on alcohol interactions with the brain endocannabinoid signaling system still is in the early stages. Chronic alcohol has been shown to increase AEA and 2-AG levels in cells and tissue ( Basavarajappa and Hungund 1999 ; Basavarajappa et al. 2000 , 2003 ). There also is evidence that chronic alcohol exposure down-regulates CB1 receptors ( Basavarajappa et al. 1998 ). Clearly, further studies are needed to explore the mechanisms through which endocannabinoids participate in the neural actions of alcohol.
Agonists for the CB1 receptor already are in use, mainly for treating chemotherapy side effects. These agonists reduce nausea induced by chemotherapy and also stimulate appetite in both chemotherapy patients and people with AIDS ( Pertwee 2005 ). Formulations of cannabis plant extracts containing THC and other cannabinoids also have been touted for treatment of multiple sclerosis and other neurological disorders ( Wade et al. 2003 ).
Targeting enzymes involved in endocannabinoid synthesis and degradation also is a promising avenue for future applied pharmacological research ( Pertwee 2005 ). For example, inhibiting the fatty acid amide hydrolase (FAAH) enzyme that metabolizes AEA prolongs the pain-reducing function of the endocannabinoid system in certain paradigms ( Hohmann et al. 2005 ), and strategies like this may be useful in treating certain neuropathic pain disorders.
Alcohol Interactions With Neurotrophins and Steroid Hormones
Alcohol has been shown to alter intracellular signaling induced by different neurotrophins. The most consistent finding is that acute exposure to alcohol inhibits the signaling and cellular effects produced by BDNF ( Davis et al. 1999 ; Fattori et al. 2008 ; Li et al. 2004 ; Ohrtman et al. 2006 ). This inhibition may contribute to the damaging effects of fetal alcohol exposure on brain development but also appears to affect neurotrophin actions in the adult animal. Studies of chronic alcohol effects on the expression and actions of this neurotrophin are more mixed (reviewed in Davis 2008 ). Chronic alcohol exposure in adult animals reportedly increases levels of BDNF mRNA in several brain regions ( Baek et al. 1996 ; McGough et al. 2004 ; Tapia-Arancibia et al. 2001 ); in a few cases, levels of the protein itself were increased ( Bruns and Miller 2007 ; Miller 2004 ; Miller and Mooney 2004 ). However, a number of other studies have reported decreases in BDNF mRNA or no effect ( Bowers et al. 2006 ; MacLennan et al. 1995 ; Miller et al. 2002 ; Pandey et al. 1999 ; Zhang et al. 2000 ). Some intracellular signaling targets downstream from the Trk receptors, most notably the Fyn and Src protein kinases, also may be targets of acute and chronic alcohol actions ( Miyakawa et al. 1997 ; Wang et al. 2007 ).
Alcohol exposure has well-known effects on the levels of the corticosteroid hormones released from the adrenal glands. Acute and chronic alcohol exposure enhances levels of cortisol (the major adrenocorticoid hormone in humans), at least in part by increasing secretion of adrenocorticotropic hormone from the pituitary gland ( Gianoulakis 1998 ; Rivier 1996 ). The hypothalamic–pituitary–adrenal (HPA) axis responsible for brain stimulation of cortisol secretion also is activated during alcohol withdrawal and may be part of a stress-like effect of withdrawal ( Adinoff et al. 1998 ). Within the brain, alcohol also appears to interact with the so-called neurosteroids, including the progesterone metabolites that can potentiate GABA A receptor function. Acute alcohol exposure enhances levels of progesterone in the blood plasma and also increases brain levels of allopregnanolone and other neurosteroids ( VanDoren et al. 2000 ), and there is evidence that this mechanism may contribute to some neurophysiological and behavioral effects of the drug ( Biggio et al. 2007 ; Morrow et al. 2001 ). All of these steroid compounds are released in response to stressful stimuli, and thus these substances may contribute to interactions between stressful environmental stimuli and the neural actions of alcohol.
Communication within the brain involves electrical activation of neurons and chemical transmission between neurons at structures called synapses. Transmission can be broken down into fast synaptic transmission mediated by LGICs and slower-developing neuromodulation mediated by GPCRs. Neurotrophins and steroid hormones also influence neuronal function by altering intracellular signaling pathways and gene expression. Extensive research has shown that many aspects of synaptic transmission are altered by alcohol at doses and brain concentrations encountered during drug ingestion. Alcohol can affect numerous neurotransmitter, neurotrophin, and steroid hormone systems in the brain to produce acute intoxication, as well as neuroadaptations that contribute to tolerance and dependence. The neurotoxic effects produced by alcohol ingestion also involve neurotransmitters. Many of the neurotransmitter systems that are implicated in alcohol actions are currently considered as potential targets for pharmacotherapeutic approaches to combating alcohol abuse and alcoholism. Articles in this two-part series will describe the specific neural effects that contribute to different alcohol actions on the nervous system. Neurotransmitters and synaptic communication, as well as neurotrophins and steroid hormones, figure prominently in these neural effects of alcohol.
1 For a definition of this and other technical terms, see the Glossary, pp. 279–283 .
2 Paracrine means that the substances are released from a given cell and produce their actions only on nearby cells (as opposed to being carried through the bloodstream to distant targets).
3 AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropi-onic acid) is a specific agonist for the AMPA receptor.
4 NMDA ( N -methyl- d -aspartic acid) is a specific agonist at the NMDA receptor and therefore mimics the action of glutamate on that receptor. In contrast to glutamate, NMDA binds to and regulates the above receptor only but not other glutamate receptors.
5 A second messenger system is a method of cellular signaling, whereby a diffusible signaling molecule is rapidly generated/released which can then go on to activate effector proteins within the cell to exert a cellular response.
F inancial D isclosure
The author declares that he has no competing financial interests.
- Adinoff B, Iranmanesh A, Veldhuis J, Fisher L. Disturbances of the stress response: The role of the hypothalamic-pituitary-adrenal axis during alcohol withdrawal and abstinence. Alcohol Research & Health. 1998; 22 (1):67–72. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Ait-Daoud N, Malcolm RJ, Jr, Johnson BA. An overview of medications for the treatment of alcohol withdrawal and alcohol dependence with an emphasis on the use of older and newer anticonvulsants. Addictive Behaviors. 2006; 31 (9):1628–1649. [ PubMed ] [ Google Scholar ]
- Alger BE. Retrograde signaling in the regulation of synaptic transmission: Focus on endocannabinoids. Progress in Neurobiology. 2002; 68 :247–286. [ PubMed ] [ Google Scholar ]
- Altshuler HL, Phillips PA, Feinhandler DE. Alteration of ethanol self-administration by naltrexone. Life Sciences. 1980; 26 :679–688. [ PubMed ] [ Google Scholar ]
- Altar CA, DiStefano PS. Neurotrophin trafficking by anterograde transport. Trends in Neuroscience. 1998; 21 (10):433–437. [ PubMed ] [ Google Scholar ]
- Amara SG, Sonders MS. Neurotransmitter transporters as molecular targets for addictive drugs. Drug and Alcohol Dependence. 1998; 51 (1–2):87–96. [ PubMed ] [ Google Scholar ]
- Arnsten AF. Stimulants: Therapeutic actions in ADHD. Neuropsychopharmacology. 2006; 31 (11):2376–2383. [ PubMed ] [ Google Scholar ]
- Ariwodola OJ, Weiner JL. Ethanol potentiation of GABAergic synaptic transmission may be self-limiting: Role of presynaptic GABA(B) receptors. Journal of Neuroscience. 2004; 24 (47):10679–10686. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Baek JK, Heaton MB, Walker DW. Up-regulation of high-affinity neurotrophin receptor, trk B-like protein on western blots of rat cortex after chronic ethanol treatment. Brain Research: Molecular Brain Research. 1996; 40 :161–164. [ PubMed ] [ Google Scholar ]
- Barbacid M. Neurotrophic factors and their receptors. Current Opinion in Cell Biology. 1995; 7 :148–155. [ PubMed ] [ Google Scholar ]
- Barde YA, Edgar D, Thoenen H. Purification of a new neurotrophic factor from mammalian brain. The EMBO Journal. 1982; 1 :549–553. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Barde YA. Neurotrophins: A family of proteins supporting the survival of neurons. Progress in Clinical and Biological Research. 1994; 390 :45–56. [ PubMed ] [ Google Scholar ]
- Barrett JE, Vanover KE. 5-HT receptors as targets for the development of novel anxiolytic drugs: Models, mechanisms and future directions. Psychopharmacology (Berlin) 1993; 112 (1):1–12. [ PubMed ] [ Google Scholar ]
- Basavarajappa BS, Hungund BL. Chronic ethanol increases the cannabinoid receptor agonist anandamide and its precursor N-arachidonoylphosphatidylethanolamine in SK-N-SH cells. Journal of Neurochemistry. 1999; 72 (2):522–528. [ PubMed ] [ Google Scholar ]
- Basavarajappa BS, Cooper TB, Hungund BL. Chronic ethanol administration down-regulates cannabinoid receptors in mouse brain synaptic plasma membrane. Brain Research. 1998; 793 (1–2):212–218. [ PubMed ] [ Google Scholar ]
- Basavarajappa BS, Saito M, Cooper TB, Hungund BL. Stimulation of cannabinoid receptor agonist 2-arachidonylglycerol by chronic ethanol and its modulation by specific neuromodulators in cerebellar granule neurons. Biochimica et Biophysica Acta. 2000; 1535 (1):78–86. [ PubMed ] [ Google Scholar ]
- Basavarajappa BS, Saito M, Cooper TB, Hungund BL. Chronic ethanol inhibits the anandamide transport and increases extracellular anandamide levels in cerebellar granule neurons. European Journal of Pharmacology. 2003; 466 (1–2):73–83. [ PubMed ] [ Google Scholar ]
- Biggio G, Concas A, Follesa P, et al. Stress, ethanol, and neuroactive steroids. Pharmacology & Therapeutics. 2007; 116 (1):140–71. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Bowers BJ, Radcliffe RA, Smith AM, et al. Microarray analysis identifies cerebellar genes sensitive to chronic ethanol treatment in PKCγ mice. Alcohol. 2006; 40 :19–33. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Bowery NG. GABA B receptor: A site of therapeutic benefit. Current Opinion in Pharmacology. 2006; 6 (1):37–43. [ PubMed ] [ Google Scholar ]
- Bowery N, Bitteger H, Olpe H-R, editors. GABA B Receptors in Mammalian Function. New York: Wiley and Sons; 1990. [ Google Scholar ]
- Brodie MS, Pesold C, Appel SB. Ethanol directly excites dopaminergic ventral tegmental area reward neurons. Alcoholism: Clinical and Experimental Research. 1999; 23 (11):1848–1852. [ PubMed ] [ Google Scholar ]
- Bruns MB, Miller MW. Neurotrophin ligand-receptor systems in somatosensory cortex of adult rat are affected by repeated episodes of ethanol. Experimental Neurology. 2007; 204 :680–692. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Brunton L, Lazo J, Parker K. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. New York: McGraw-Hill; 2005. [ Google Scholar ]
- Charness ME. Ethanol and opioid receptor signalling. Experientia. 1989; 45 :418–428. [ PubMed ] [ Google Scholar ]
- Chao MV, Hempstead BL. p75 and Trk: A two-receptor system. Trends in Neuroscience. 1995; 18 :321–326. [ PubMed ] [ Google Scholar ]
- Chevaleyre V, Takahashi KA, Castillo PE. Endocannabinoid-mediated synaptic plasticity in the CNS. Annual Review of Neuroscience. 2006; 29 :37–76. [ PubMed ] [ Google Scholar ]
- Choi DS, Cascini MG, Mailliard W, et al. The type 1 equilibrative nucleoside transporter regulates ethanol intoxication and preference. Nature Neuroscience. 2004; 7 (8):855–861. [ PubMed ] [ Google Scholar ]
- Colombo G, Orru A, Lai P, et al. The cannabinoid CB1 receptor antagonist, rimonabant, as a promising pharmacotherapy for alcohol dependence: Preclinical evidence. Molecular Neurobiology. 2007; 36 (1):102–112. [ PubMed ] [ Google Scholar ]
- Compton WM, Volkow ND. Major increases in opioid analgesic abuse in the United States: Concerns and strategies. Drug and Alcohol Dependence. 2006; 81 (2):103–107. [ PubMed ] [ Google Scholar ]
- Connor M, Christie MD. Opioid receptor signalling mechanisms. Clinical and Experimental Pharmacology & Physiology. 1999; 26 (7):493–499. [ PubMed ] [ Google Scholar ]
- Coutinho V, Knöpfel T. Metabotropic glutamate receptors: Electrical and chemical signaling properties. Neuroscientist. 2002; 8 (6):551–561. [ PubMed ] [ Google Scholar ]
- Davis MI. Ethanol-BDNF interactions: Still more questions than answers. Pharmacology and Therapeutics. 2008; 118 (1):36–57. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Davis MI, Szarowski D, Turner JN, et al. In vivo activation and in situ BDNF- stimulated nuclear translocation of mitogen-activated/extracellular signal-regulated protein kinase is inhibited by ethanol in the developing rat hippocampus. Neuroscience Letters. 1999; 272 :95–98. [ PubMed ] [ Google Scholar ]
Curious Kids: how does our brain send signals to our body?
Senior Lecturer in Developmental Neuroscience, University of Central Lancashire
Disclosure statement
Georgia Chronaki does not work for, consult, own shares in or receive funding from any company or organisation that would benefit from this article, and has disclosed no relevant affiliations beyond their academic appointment.
University of Central Lancashire provides funding as a member of The Conversation UK.
View all partners
How does our brain send signals to our body? – Aarav, aged nine, Mumbai, India.
For hundreds of years, scientists have tried to understand the human brain – known as the most complex organ in the universe.
The average human brain contains about 86 billion nerve cells, called neurons. These are the building blocks of your brain. Neurons communicate with each other by sending chemical and electrical signals.
Each neuron is connected with other neurons across tiny junctions called “synapses”. Impulses rush along tiny fibres, like electrical wires, from one neuron to the next.
Every time you recognise a familiar face, hear a voice, learn something new or read a word like this, millions of neurons are communicating with each other through hundreds of millions of synapses.

Curious Kids is a series by The Conversation , which gives children the chance to have their questions about the world answered by experts. If you have a question you’d like an expert to answer, send it to [email protected] . We won’t be able to answer every question, but we’ll do our very best.
Making sense
The brain is the body’s control centre: it sends messages to your body through a network of nerves called “the nervous system”, which controls your muscles, so that you can walk, run and move around.
The nervous system extends through your body from your spinal cord, which runs from your brain down your backbone, like the branches of a tree.
Read more: Curious Kids: how can we see what we are imagining as well as what's in front of us?
The brain is also in charge of the way you experience the world around you. Imagine you’re walking in a forest. The light bouncing off the trees enters your open eyes; the chirping sounds of the birds reach your ears; and the damp smell of the forest soil wafts up your nose.

The nerve cells in your eyes, ears and nose detect these sensations, and send signals to different parts of your brain, which turn them into what you see, hear and smell – all in a matter of milliseconds.
Sending signals
As well as sending electrical signals through the nervous system, the brain also uses chemical signals to control processes in the body.
Have you ever wondered why you feel sleepy? When the sun goes down, a part of your brain called the pineal gland produces a hormone called melatonin, which makes you feel tired.
Melatonin is produced a few hours later in teenagers than it is in adults and children. This makes teenagers want to go to bed and wake up later than adults and children.
Read more: The biological reason why it's so hard for teenagers to wake up early for school
Teenagers are not lazy : it’s all to do with how the brain sends signals to the body.
A sense of self
As well as allowing us to move around and understand what’s going on in the world, the brain gives us a sense of who we are – a “sense of self” , that’s different to other people.
Scientists don’t quite yet understand how the brain creates each person’s sense of self. But research has shown that when people think they are being watched by others, certain parts of their brains are busy. This part – called the “medial prefrontal cortex” – is what makes you feel self-conscious.
The human brain, with its billions of neurons working together, is sending signals to your body to determine how you feel, from one moment to the next.
The brain is always trying to find ways to explain the sensations that we feel in our body. And the same sensation can have different meanings in different contexts. Here’s an example: when you see a delicious piece of cake and your stomach churns, your brain might send signals to your body that you’re hungry and excited.

But if you’re about to have a test at school, your brain may give a different meaning to that churning stomach and create feelings of fear or anxiety.
The experiences you have become part of how your brain makes sense of what is happening to you. This means that people have more control over their emotions than they might think, because the brain can learn how to respond to experiences differently. As author Wayne Dyer said : “If you change the way you look at things, the things you look at change.”
From perceiving the world through the five senses, to creating our sense of self, uncovering how the human brain sends signals to our body is one of the biggest mysteries in science.
Children can have their own questions answered by experts – just send them in to Curious Kids , along with the child’s first name, age and town or city. You can:
- email [email protected]
- tweet us @ConversationUK with #curiouskids
- DM us on Instagram @theconversationdotcom
Here are some more Curious Kids articles, written by academic experts:
How can we see what we are imagining but still see what’s in front of us? – Malala Yousafzai class, Globe Primary School, London, UK.
Why is the sea salty? – Torben, aged nine, Sussex, UK.
Why do I have boogies and why does my nose keep replicating them? – Duncan, aged seven, Sydney, Australia.
- Neuroscience
- Curious Kids
- Articles for young people

Service Centre Senior Consultant

Director of STEM

Community member - Training Delivery and Development Committee (Volunteer part-time)

Chief Executive Officer

Head of Evidence to Action

COMMENTS
An Overview of the Different Parts of a Neuron
How do neurons communicate (so quickly)?
The nerve cell, or neuron, is the key player in the activity of the nervous system. It conveys information both electrically and chemically. Within the neuron itself, information is passed along through the movement of an electrical charge (i.e., impulse). The neuron has three main components: (1) the dendrites, thin fibers that extend from the ...
Neurons Transmit Messages In The Brain
12.4 Communication Between Neurons - Anatomy & Physiology
Temporary changes to the cell membrane voltage can result from neurons receiving information from the environment, or from the action of one neuron on another. These special types of potentials influence a neuron and determine whether an action potential will occur or not. Many of these transient signals originate at the synapse. Graded Potentials
How Neurons Communicate
The movement of signals between neurons (article)
Light causes channels to open in these different light-detecting neurons, which sends an electrical message to the synapses of neurons inside your brain (Fig. 3). Signals travel along the optic nerve to carry information into your brain . This information is then processed in visual center synapses to interpret the light images.
Two different strategies are responsible for electrical communication between neurons. One is the consequence of low resistance intercellular pathways, called "gap junctions", for the spread of electrical currents between the interior of two cells. The second occurs in the absence of cell-to-cell contacts and is a consequence of the ...
Overview of neuron structure and function (article)
Anatomy of a neuron (video) | Human biology
Nerve cells generate electrical signals that transmit information. Although neurons are not intrinsically good conductors of electricity, they have evolved elaborate mechanisms for generating electrical signals based on the flow of ions across their plasma membranes. Ordinarily, neurons generate a negative potential, called the resting membrane potential, that can be measured by recording the ...
Brain cells function using rapid electrical impulses, a process that underlies our thoughts, behavior, and perception of the world. Yet, for a long time, it's been challenging for scientists to see exactly how individual neurons work together in larger circuits. Now, a new technique reported in Nature finally gives the clearest picture ever ...
35.2 How Neurons Communicate - Biology 2e
Neurons receive chemical input from other neurons through dendrites and communicate information to other cells through axons. Neurons also are "excitable" cells. The neuronal surface membrane contains an abundance of proteins known as ion channels that allow small charged atoms to pass through from one side of the membrane to the other.
Electrical impulses travel through neurons. Giovanni Canchemi/Shutterstock. ... Teenagers are not lazy: it's all to do with how the brain sends signals to the body. A sense of self.