My Account / Create Account
Forgot My Password

PracticeMatch Physician Articles
What to expect from your site qualification visit: last minute prep tips.
Site Qualification visits are an essential component of the clinical trials site selection process. This visit allows both you and the trial's Sponsor to learn more about each other and ascertain if the study is a good fit. It is also your chance to show that you and your facility can support the many regulatory and performance requirements that each clinical study demands.
Do you know what to expect during your site visit? Learn more about how you can prepare with these easy tips:

Preparing for the Sponsor
Assessing the suitability of your staff and site is a top priority for Sponsors during a site visit. Sponsor representatives will review the qualifications and background of all participating physicians and staff at your clinical site. They will also assess whether you have sufficient training, time and resources to fulfill the clinical study's requirements.
Prepare for these reviews by:
Having updated and comprehensive CV's on hand for the Sponsor to review for key study personnel. It is important to emphasize any prior clinical trial work or publications. This can also include experience gained while in Residency training. Ensure that you also have copies of applicable medical licenses on hand for review during and following the site qualification visit.
Holding a group protocol review meeting with your staff prior to the site qualification visit. Be sure to include all study staff and discuss the protocol, IRB requirements, SOPs, and any required training in depth. Try to brainstorm any issues that may arise while trying to execute the protocol, as this can help generate points that may need clarification during the visit. Sponsors are looking for experienced, efficient staff members who are engaged and will make their study a priority.
Ensuring you have a recruitment strategy and enrollment target timeline ready ahead of time to share with the Sponsor. Sponsors want to know that you will be able to meet their recruitment goals, so it is key to show that you are prepared with a strategy and have contingency recruitment plans.
Preparing for the Site Qualification Facility Inspection
An important part of any site qualification visit involves inspecting available clinical and storage space. Clinical trials require dedicated areas for study administration, examinations, drug and data storage. Sponsors will assess whether you have the necessary equipment and space to execute their study requirements.
Be sure that all designated areas are used exclusively for their assigned purposes. Prior to inspection, ensure that all areas are clean and have only applicable items stored in the area.
Ensure all applicable laboratory and medical licenses are up to date prior to the start of the site qualification visit.
Check that your facility has met the necessary security requirements in regard to drug storage, data security and access.
Add Your Heading Text Here
Clinical site qualification visits best practices.

Medha Datar
- March 12, 2023

Clinical site qualification visits are an essential step in ensuring that clinical trial sites are qualified to conduct a study. These visits allow sponsors and contract research organizations (CROs) to assess the suitability of potential sites and verify that they meet the study requirements.

Here are some best practices for conducting clinical site qualification visits:
- Prepare in advance
Preparation is critical to the success of a site qualification visit. Before the visit, review the study protocol, site-specific documents, and any other relevant study materials to understand the site’s capabilities and needs. This will help you prepare a list of questions and requirements that need to be addressed during the visit.
- Establish clear objectives
It is essential to establish clear objectives for the visit and communicate them to the site staff. Make sure they understand the purpose of the visit and what is expected of them. Clear communication will ensure that the visit runs smoothly and that the site staff is fully engaged in the process.
- Conduct a pre-visit assessment:
Conduct a pre-visit assessment to gather information about the site’s history, experience, infrastructure, and staffing. This will help you identify any potential issues that need to be addressed during the visit.
- Plan the visit itinerary
It is crucial to plan the itinerary of the visit to ensure that all necessary areas are covered. Schedule time to meet with key staff members, review documentation and tour the site facilities. A well-planned itinerary will help ensure that the visit is efficient and that all relevant areas are evaluated.
- Conduct an opening meeting
Conduct an opening meeting to introduce the site staff to the study team and clarify the objectives of the visit. This meeting should include a review of the study protocol, site-specific documents, and any relevant study materials. The meeting is an opportunity to establish rapport with the site staff and to build a positive working relationship.
- Review documentation
Review the site’s regulatory and institutional review board (IRB) documentation to ensure that it is up to date and in compliance with local regulations. This includes reviewing the informed consent forms, investigator brochures, and other relevant documents. The review will help ensure that the site is qualified to conduct the study and that all regulatory requirements have been met.
- Assess facilities and equipment
Evaluate the site’s facilities and equipment to ensure that they meet the study requirements. This includes reviewing the storage, security, and access control measures in place for investigational products and study documents. The assessment will help ensure that the site is equipped to handle the study materials and that they are being stored and secured appropriately.
- Assess site personnel
Assess the qualifications and experience of the site personnel, including the principal investigator, sub-investigators, research coordinators, and study nurses. Verify that they have received appropriate training and are familiar with the study procedures. The assessment will help ensure that the site staff is qualified to conduct the study and that they have the necessary experience to manage the study requirements.
- Evaluate data management procedures
Evaluate the site’s data management procedures, including the use of electronic data capture (EDC) systems, source documentation, and quality control measures. The evaluation will help ensure that the site is equipped to manage the study data and that the data is being collected and managed accurately and securely.
- Conduct a closeout meeting
Conduct a closeout meeting to summarize the findings of the visit and provide feedback to the site staff. Clarify any deficiencies and outline the steps that need to be taken to address them. The meeting is an opportunity to ensure that the site staff understands the findings and to establish a plan of action to address any deficiencies.
Follow up with the site staff after the visit to ensure that any deficiencies have been addressed and that the site is ready to proceed with the study. Continue to monitor the site throughout the study to ensure that it is meeting the study requirements. Ongoing communication with the site staff is critical to the success of the study.
In conclusion, site qualification visits are an essential
By following these best practices, sponsors and CROs can conduct effective and efficient clinical site qualification visits that ensure the quality of the study data and the safety of the study participants.
Cloudbyz Unified Clinical Trial Management (CTMS) is a comprehensive, integrated solution to streamline clinical trial operations. Built on the Salesforce cloud platform, our CTMS provides real-time visibility and analytics across study planning, budgeting, start-up, study management, and close-out. Cloudbyz CTMS can help you achieve greater efficiency, compliance, and quality in your clinical operations with features like automated workflows, centralized data management, and seamless collaboration. Contact us today to learn how Cloudbyz CTMS can help your organization optimize its clinical trial management processes.
To know more about the Cloudbyz Unified Clinical Trial Management Solution contact [email protected]
Request a demo specialized to your need.
Subscribe to our weekly newsletter

At Cloudbyz, our mission is to empower our clients to achieve their business goals by delivering innovative, scalable, and intuitive cloud-based solutions that enable them to streamline their operations, maximize efficiency, and drive growth. We strive to be a trusted partner, dedicated to providing exceptional service, exceptional products, and unparalleled support, while fostering a culture of innovation, collaboration, and excellence in everything we do.
Subscribe to our newsletter
CTMS CTBM eTMF EDC RTSM Safety & PV PPM
Business Consulting Services Professional Services Partners CRO Program
Blog e-Books & Brochures Videos Whitepapers News Events
About us Careers Sustainability FAQ Terms & Conditions Privacy Policy Compliance
© 2024 Cloudbyz
StudyOrganizer – Your Complimentary Study Management Assistant
Clinical Trial Monitoring | In-Person and Remote
Since 2020, clinical research teams have radically changed how they approach clinical trial monitoring–and researchers need to adapt to this change to avoid being left behind.
Before the pandemic, the most common type of monitoring was in-person monitoring, where Clinical Research Associates traveled by car or plane to each site to view their documents and facilities. But now, 97% of clinical trial sponsors use technology to review a study’s source data and documents remotely.
So what does remote monitoring look like? In the past, sponsors and Contract Research Organizations (CROs) often forced research sites to upload regulatory documents and source data into a sponsor portal. The sponsor or CRO would then log into the portal to view the documents.
But this strategy caused extra work for research sites. Sites had to compile their electronic Investigator Site File (eISF), then re-upload all of those documents into the portal. That’s why, in 2022, 63% of sponsors chose to deploy electronic Investigator Site Files that sites were able to own. Sites could set up the eISF according to their preferences, manage documents and data inside it, and then grant sponsors access for remote monitoring.
In 2023, 78% of sponsors plan to use a site-friendly eISF for remote monitoring. It’s now critically important for sponsors, sites, and CROs to understand how remote monitoring is different from in-person monitoring and how to use remote monitoring most effectively.
Keep reading to learn more about the different types of clinical trial monitoring and how your clinical research team can use them effectively.
Traditional Clinical Trial Monitoring vs. Remote Monitoring
The traditional method of clinical trial monitoring begins with the Clinical Research Associate boarding a plane or driving to the site. The research site must prepare for their visit by setting aside a quiet room and filling it with paper binders or a computer where the monitor can observe them working on documents.
The CRA reviews as many of the documents as they can during their prescribed visit, leaves feedback for the site, and moves onto the next site. The research site then has to scramble to correct any flaws the CRA found and wait months or even a year before receiving any more feedback.
With remote monitoring, the CRA reviews sites’ documents and data online through a Site Enablement Platform, like an eISF or eRegulatory platform. They then provide feedback for the site, who can quickly incorporate it. When or if in-person visits take place, the CRA can spend their time evaluating the site’s facilities and getting to know the staff instead of sitting in a room and staring at documents.
Types of Clinical Trial Monitoring Visits
Many types of monitoring visits occur throughout the course of a trial, and most of them can be performed remotely.
Here are a few important types of clinical trial monitoring visits and how they change when they’re remote instead of in-person:
Site Qualification Visit (SQV)
During this visit, the sponsor or CRO evaluates the investigator’s credentials and the site’s resources and decides whether the site has the ability to conduct a study. This visit takes place after the feasibility assessment and after the site has received a protocol, synopsis, and Confidential Disclosure Agreement.
The site can provide the information for this visit remotely, saving CRAs from traveling to sites that may not be a good fit for the trial.
Sites can use a Site Enablement Platform to share:
- Investigator credentials
- Site staff and Clinical Research Coordinator credentials
- Demographics of their patient population
- Information on their facilities
- Historical site data
- Regulatory documents
By sharing these documents through an eISF or eRegulatory platform, sites have control over the story they share with sponsors or CROs–and sponsors and CROs can learn about the site without wasting their CRAs’ time .
Site Initiation Visit (SIV)
The Site Initiation Visit (SIV) takes place after a site is selected for a study but before patients are enrolled. The CRA uses this visit to give site staff and investigators specific protocol information and to review regulatory forms.
Parts of the SIV that the site can do remotely include:
- Signing and submitting regulatory forms
- Completing protocol and study-specific training
- Reviewing protocols
- Setting up logs
- Confirming the supplies for the trial have been received
- Communicating with the sponsor or CRO with any questions
When the site performs these tasks online, they won’t have to waste time preparing for a visit about forms and training they’ve already completed. This strategy leads to faster study start-up times.
Interim Monitoring Visit (IMV)
Interim Monitoring Visits take place multiple times throughout the study–but using traditional methods, CRAs can only visit about one site per week. It would take one CRA an entire year to visit roughly 50 sites–and to make up the difference, sponsors and CROs have to hire more CRAs, a goal rendered difficult by staffing shortages .
With remote clinical trial monitoring, 75% of CRAs visit 2-5 sites per week, increasing their efficiency for IMVs by 2-5x.
CRAs can use a Site Enablement Platform to remotely:
- Review Drug Accountability Forms, Case Report Forms (CRFs), and logs
- Access dashboards that show how the study is progressing at each site
- Assign follow-up tasks
Then, when CRAs perform in-person visits, they can spend their time meeting staff, exploring patient recruitment and experience strategies, and checking on protocols–not reviewing documents.
Closeout Site Visit (COV)
The closeout site visit, which takes place when a site either completes or withdraws from a study, is one of the easiest monitoring visits to complete remotely . All of the relevant documents and data for closeout will already be in the eISF or eRegulatory platform. The site simply has to make sure they’re complete and send them to the sponsor.
The sponsor or CRO can then remotely:
- Route all relevant documents to the eTMF
- Archive documents in long-term cold storage for 25 years
Closeout needs to be compliant, but the more efficient it is, the more quickly a trial can be completed and the sponsor can send the results to regulatory authorities.
Benefits of Remote Monitoring
When the Florence team asked sponsors why they adopted technology in 2022, the top answer was to enable remote monitoring and regulatory document access. The number-two reason was enabling remote source access.
Why have remote source access, document access, and monitoring become so popular? The answer is simple: these strategies save sponsors, CROs, and sites time.
Benefits of Remote Monitoring for CRAs
Clinical Research Associates (CRAs) used to spend 18% of their time traveling. That number only includes time spent on planes or in cars, not time spent on site or reviewing regulatory documents. Remote clinical trial monitoring can save CRAs up to 7.5 hours a week in travel time alone.
One survey of CRAs showed that without remote monitoring, most CRAs spent more than 7 hours reviewing documents per site. With remote monitoring, most CRAs only spent 3-4 hours of their on-site time reviewing documents.
But remote monitoring doesn’t only make clinical trial monitoring faster: a survey of CRAs showed that it can also make monitoring more accurate.
- 78% of CRAs can move documents into the eTMF faster
- 76% can monitor sites more often
- 75% can identify potential risks with site documents earlier
- 68% can remediate document issues faster
- 66% can spend less time on repetitive tasks
Remote monitoring also gives CRAs the opportunity to help more of their sites. Without remote monitoring, 52% of CRAs only monitored one site per week. With it, 75% of CRAs were able to monitor 2-5 sites per week–more than doubling their efficiency. To learn more about the role of a CRA, check out our Impact of Remote Site Monitoring on CRAs Benchmark Report.
Benefits of Remote Monitoring for Site Staff
Remote clinical trial monitoring through an eISF or another Site Enablement Platform can also help sites. However, Site Enablement Platforms, like eISFs and eRegulatory platforms, need to match site workflows.
A fully-equipped eISF or eRegulatory platform can hold:
- Regulatory forms
- Imported source data
- Informed consent documents
An excellent Site Enablement Platform will also help sites create, edit, and sign documents electronically, establish document workflows, assign tasks to stakeholders, set up users, and give their sponsors permission to view and edit documents.
Sites will no longer have to manage documents in a paper binder or different software platform, then turn around and upload the completed documents to a sponsor portal–a process that wastes time sites could be spending on patients or essential trial tasks.
To help you evaluate different Site Enablement Platforms, download our checklist that includes considerations about site workflows and remote capabilities.
Benefits of Remote Monitoring for Site-Sponsor Collaboration
With a Site Enablement Platform , sites won’t have to spend hours preparing for clinical trial monitoring visits by setting up a quiet room filled with binders or computers. CRAs also won’t have to travel to a different city solely to look at documents in an enclosed room.
Instead, site staff can redact any Protected Health Information (PHI) from their documents and data, then give monitors controlled remote access to their eISF (at Florence, we call this feature the Monitor Review Module .)
Before they visit the site, CRAs can:
- Look at source data and regulatory documents and leave comments for the site
- See if the site incorporated needed changes
- Route documents to the eTMF
Sites can then use in-person visits to get to know their CRAs, show off their facilities, and focus on patient recruitment strategies.
But if remote monitoring is going to work, it has to be compliant with clinical trial regulations. Luckily, this is possible.
Compliance and Remote Monitoring
The FDA has released guidance that allows centralized remote monitoring , as long as sponsors, sites, and CROs continue to follow all clinical trial regulations.
ICH E8 R1 , the set of international guidelines that many countries rely on when building their clinical trial regulations, also allows flexibility in monitoring, including using centralized remote monitoring. (You can learn more about ICH E8 R1 here.)
However, sites, sponsors, and CROs who embrace remote clinical trial monitoring will still need to follow electronic document regulations like 21 CFR Part 11 and Annex 11 , as well as privacy regulations like GDPR , HIPAA, and CCPA .
Every Site Enablement Platform should have the following features to ensure compliant remote monitoring:
- Document audit trails
- Document version control
- User roles and permissions
- Digital cold storage of documents and data for 25 years
- Redaction features for PHI
With a compliant eISF or eRegulatory platform, sites, sponsors, and CROs can turn many of their clinical trial monitoring visits – from Site Qualification to Closeout – into remote visits.
To learn more about how a Site Enablement Platform can accelerate your study while keeping it compliant, check out our remote monitoring case study .
Exploring Remote and In-Person Clinical Trial Monitoring
In-person monitoring can help build connections between sites and their sponsors. But holding every monitoring visit in person forces CRAs to spend almost 20% of their time traveling instead of working on trial-related activities.
In-person monitoring also costs time for sites, who must interrupt their regular patient care activities to set up rooms, gather documents, or provide access to their computers.
With a Site Enablement Platform designed for site workflows, sites can maintain their documents and data throughout the trial. Then, when they’re ready for remote monitoring visits, they can simply give CRAs controlled access to their documents and data.
With remote monitoring, CRAs can check in on their sites whether they’re at the office, at home, or on their way to another site. And trials can keep moving, bringing new treatments to the patients waiting for them.
If you’d like to learn more about increasing the efficiency of monitoring visits, check out our Complete Guide to Clinical Trial Monitoring , with specific tips on how monitoring can be performed in-person and remotely.
Get in Touch
Global offices.
USA (HQ) 600 Peachtree St. NE, Suite 920 Atlanta, GA 30308
Serbia Bulevar Kralja Aleksandra 84 11000 Belgrade, Serbia
© 2023. Florence Healthcare.

Providing courses, products & services so Clinical Research Professionals can thrive & achieve fulfilling careers.
How to Plan a Productive Site Monitoring Visit
by ClinEssentials Team | Feb 28, 2022 | Tools for Research Professionals , Clinical Research Associates , Clinical Research Coordinators , Tips for Research Professionals | 0 comments

Many Clinical Research Associates are unprepared for their clinical trial monitoring visits, which is an important part of their role. It is understandable – CRAs are busy and it can be hard to find time for planning where there are so many other tasks to complete right away.
Monitoring visits are when CRAs ensure their studies are staying in compliance and on track. Creating a plan for the allotted time is the best way to ensure a productive, efficient monitoring visit.
What Happens at a Clinical Trial Monitoring Visit
Monitoring visits are typically very full days – jam-packed with all of the action items the CRA and the site need and want to accomplish.
A CRA may visit a site for site initiation, an Interim Monitoring Visit (IMV) – also called a Routine Monitoring Visit (RMV), or a study close-out. IMVs are the most common and usually happen every 4-6 weeks. However, monitoring plans differ and are determined by the Sponsor or CRO before a trial begins.
3 objectives of a monitoring visit
While there are many things that a CRA wants to successfully complete at a monitoring visit, there are three main objectives for each visit:
- Discover any data errors and/or discrepancies
- Evaluate the site staff’s understanding of the protocol and procedures
- Determine compliance
Standard tasks, meetings and goals of a monitoring check-in visit
Tasks are based on the reason for the visit. Since an Interim Monitoring Visit is the most common, here are the general business items that a CRA will address at a monitoring check-in visit:
- Review the regulatory binder, study documentation, and CRF entries
- Audit screening, enrollment, visit, and follow up data
- Conduct Source Data Verification
- Perform a safety assessment
- Examine the investigational product and study supplies
- Evaluate study personnel and site facilities
- Meet with the Principal Investigator and site staff
The Association of Clinical Research Professionals (ACRP) created this extensive checklist of tasks for a monitoring visit .
CRAs are responsible for documenting everything they observe, notice, and discuss during a visit. Following the visit, this information is compiled into a clinical monitoring report. Here are 5 guidelines for writing a useful clinical monitoring report from MasterControl.
Consequences of being Unprepared for a Monitoring Visit
Being prepared for a monitoring visit is instrumental in the success of the visit. When both the Clinical Research Associate and the research site are prepared, monitoring visits run smoothly and efficiently. The patient volunteers, clinical research team, and CRO or Sponsor are all negatively affected when the CRA or the site staff has not taken the time to prepare for a monitoring visit.
Scheduling conflicts and unavailability of key team members can lead to important meetings that have to wait until the next visit.
The site may not have the necessary documents or the regulatory binder ready for the CRA to review, which is one of the primary activities for most monitoring visits.
When monitoring visits are not successful and all essential tasks and meetings do not occur, the trial can be delayed – and potentially over budget. Sponsors and CROs may lose trust in the site and the staff.
3 Steps for a Successful Monitoring Visit
With proper communication and a simple 3 step process, preparing for a monitoring visit does not have to be overly time-consuming. Plus, spending time in advance will be time saved through an efficient, organized visit!
- Plan the visit. Download this free checklist that will lead to a well-planned visit. It even includes a list of documents to have on your computer and in your work bag. (The checklist can be modified based on the protocol or directions from the research team.)
- Review the study protocol and have a thorough understanding of the entire document.
- Bring clinical trial monitoring tools! Use the Visit To Do List to stay focused and on task. Provide the site staff with a copy of the Action Item List – and use the other copy to write the Monitoring Visit Report and Follow-Up Letter.
For more tips, check out Dan Sfera’s YouTube video about how CRAs should prepare for a site monitoring visit .
Stop showing up unprepared for a monitoring visit! As a leader and essential member of the research team, the site staff will appreciate and respect you for your thoroughness and organization – and this will be evident in their work!
For more great ideas and monitoring tools that help Clinical Research Professionals become more efficient, visit www.clinessentials.com .
Submit a Comment Cancel reply
Your email address will not be published. Required fields are marked *
Submit Comment
More Resources

How to Get into Clinical Research
by ClinEssentials Team | Feb 7, 2024 | Clinical Research Careers , Clinical Research Coordinators , Clinical Trial Assistants , Tips for Research Professionals

How Clinical Trial Managers Are Involved with Study Recruitment
by ClinEssentials Team | Jan 10, 2024 | Clinical Research Careers , Clinical Trial Managers , Tips for Research Professionals

CRA Responsibilities for Each Stage of a Clinical Trial
by ClinEssentials Team | Dec 27, 2023 | Clinical Research Careers , Clinical Research Associates , Tips for Research Professionals

How to Review a Clinical Trial Protocol (Free Checklist Included)
by ClinEssentials Team | Nov 8, 2023 | Clinical Research Careers , Clinical Trial Managers , Tips for Research Professionals , Tools for Research Professionals
- Global Reach
- Leadership Team
- Mission, Vision, & Values
- Testimonials
- Emerging Technology and Product Development Strategy
- Data Analytics, Safety, and Compliance
- Clinical Operations
- Market Access
- Therapeutic Expertise
- Medical Device
- Medical Technology
- In Vitro Diagnostics (IVD)
- Biotech / Biologics
- Knowledge Library

How to Successfully Perform Remote Monitoring During the COVID-19 Pandemic
April 2, 2020.
Share On Share on LinkedIn Share on X (Twitter) Share on Facebook
Site Qualification Visit (SQV)
Engage the client in evaluating the benefit of having a qualification visit. SQVs can be performed remotely with the support of other factors and documentation. If need be, waiving the formal visit may be an option if procedures and policies allow.
Items to consider:
- e.g., investigator experience, time and resources to perform the study, facilities, identified staff
- Conceptualize all site spaces for the designated study that a monitor would need to tour to think of key questions to ask regarding site facilities. (e.g., “Is the monitoring space adjacent to IP storage or is it in another building a 10-minute walk away?”)
- Use internal CTMS system data to collect intel on the site, including past experiences and results.
- In lieu of a CTMS system, reach out to coworkers who may have had first-hand experience with a site and can provide important information that is otherwise garnered from an in-person visit. They can often assist in confirming questionnaire responses by drawing upon their real-life experience.
- Work within individual site restrictions and policies to see if a remote SQV is possible.
- As physicians are in high demand, try to limit the PI portion of the SQV to 15–20 minutes, with a focus on the PI’s responsibilities and enrollment requirements. Study coordinators can cover the remaining information of the study startup process and remaining logistical information.
Site Initiation Visit
Work with the client to see where overarching procedures and policies have flexibility and come up with creative strategies for overcoming current obstacles.
SIV Presentation
Utilize platforms such as Microsoft Teams or Webex to conduct a remote conference call and present slides to the study team.
Device or Product Training
- In some cases, remote training may be feasible and can be completed.
- If device or product training needs to be in-person, consider initiating a site with the caveat that they are not allowed to enroll (or implant, or perform procedures) until formal in-person training is completed and documented in the site initiation visit report, or activation checklist, etc. In some cases, remote training may be feasible and can be completed.
Regulatory Documents
- Provide site coordinators with regulatory document templates necessary for site activation before the scheduled SIV in order to have all required signatures collected before or at the time of the remote SIV. Signatures may be collected as wet ink signatures or digitally, working with the CTAs to have them sent out using DocuSign.
Site Reference Manuals and Site Binder Materials
- Instead of supplying binders filled with the template logs, charts, protocol, case report forms, source document worksheets, and everything else that study personnel would want and need to keep on file for reference for a trial, consider supplying the documents through a secure virtual space where study personnel can access and download as needed (SharePoint or Microsoft Teams).
- When supplying study materials in this manner be sure to confirm with site, ahead of the scheduled visit, any electronic transmission issues due to institutional firewalls (e.g., site cannot utilize USBs, zip files, etc.).
- Consider supplying the training logs (and any other forms needed at the SIV to complete the site activation checklist) to the site in a pre-populated format to avoid any confusion or further delay in activation while in the remote setting.
Connect with the site to be sure they are able to complete a remote SIV. Sites have different regulations that must be followed, and they can vary from site to site — plus they may change as institutions work toward remote solutions at this time.
Interim Monitoring Visit
Develop contingency plans with the sponsor, taking into account potential limitations. Potential limitations may include:
- Access to EMR
- Access to study documents
- Bandwidth of site personnel to redact documents or provide remote access
- Data entry abilities
- Critical variables
- Patient safety
A full or partial remote monitoring visit may be appropriate if the site has the following:
- Access to study records
- Access to regulatory records
- Bandwidth to redact source documents (or provide monitor with remote access to records, e.g., direct access to EPIC, etc.)
- If critical variables have not been identified, work with the client to pinpoint them.
- Revisit the variables for visits and make a determination, based on what might be considered standard of care versus research-related only, as to which variables you may be able to anticipate being available, if any.
- Are study personnel still on-site?
- Do study personnel or monitors have remote access to the EMR system?
- What is the institution’s policy on remote monitoring and sharing redacted source?
- Was is the bandwidth available to provided resources to supply redacted source if needed?
- Tip : Always have a conversation with the sponsor regarding additional tasks for the sites if not previously agreed upon.
- Work with the source documentation that is available.
- Continue to narrow critical information — If site is limited, then maybe a narrowing of information to monitoring means changing from a focus on critical variable monitoring to a focus on eligibility criteria and safety oversight. Furthermore, a partial remote visit could even be performed on the redacted adverse event paperwork completed for patients thus far.
Even though a monitoring plan may dictate when a visit should occur doesn’t mean that, given the current health landscape and action plans in place to mitigate risk and place patient safety first, a visit is warranted. Sponsors may ask that visits be placed on hold. When making this decision with the sponsor, take into consideration:
- How many currently enrolled patients are able to continue with follow-up visits?
- Do site personnel have the bandwidth/tools/resources to complete data entry to be monitored?
- What is the risk of placing these visits on hold in terms of patient safety?
A remote visit allows us to maintain consistency and site oversight, but in the event that the decision is made to hold the visit, ensure to maintain a means of contact and “monitoring.”
- Touch-base phone call(s)
- Regular email conversations with attachments if allowed
- Query EDC CRF entries as applicable
Maintaining communication regardless of a temporary change to monitoring procedures is crucial. All efforts should be made to monitor patient safety.
Health facilities are continuing to work on tackling these obstacles and unveiling new solutions and policies every week. It is important to stay in touch and be aware so that if and when a site that was previously unable to participate in remote IMVs suddenly has a new system in place that is validated for uploading redacted source and ready for use, you know about it and can expand your monitoring capabilities.
Tip : Create a COVID-19 study tracker with all sites listed and the restrictions currently in place. Update regularly as you stay in contact with all sites. Use this tool to stay up to date on their ability to participate in completion of remote monitoring and standard trial practices otherwise completed regularly.
Back to Blog
Your product is important, and your trial deserves the team that has what it takes. It takes knowledge. It takes passion. It takes commitment. It Takes Avania.
- ITHS SharePoint
Search the Site
Connect with us, need help have a question.
Contact the Research Navigator
ITHS Email Updates

Pre-Study Preparation
The key decisions involved with the pre-study preparation of industry-sponsored clinical trials include the following:
Confirm Research Support Services/Facilities
Based on your final budget and contract, contact the supporting departments who will be involved with the trial to let them know you are ready to begin. This might include:
ITHS CLINICAL RESEARCH CENTER
ITHS RESEARCH COORDINATION CENTER
INVESTIGATIONAL DRUG SERVICE PHARMACY Harborview Medical Center (206) 744-5448 | [email protected] UW Medical Center (206) 598-6054 | [email protected] Website
UWMC LABORATORY MEDICINE Administration (206) 598-6131 | [email protected]
RESEARCH TESTING SERVICE (206) 616-8979 | [email protected]
UW DEPARTMENT OF PATHOLOGY NWBioSpecimen
UW DEPARTMENT OF RADIOLOGY RESEARCH PROGRAM
Site Initiation Visit
Many industry sponsors/Clinical Research Organizations conduct a Site Initiation Visit (SIV) to prepare and set up a research site, conduct protocol training, and ensure the Principal Investigator (PI) fully understands all trial responsibilities. The visit usually occurs after the site has completed all regulatory requirements, including Institutional Review Board approval, but prior to recruiting participants. The sponsor/Clinical Research Organization will want to meet with the PI and as many members of the research team as possible. The sponsor/Clinical Research Organization may ask to meet with representatives from supporting departments (e.g., pharmacy, radiology, lab medicine).
Topics of discussion during the site initiation visit include:
- PI responsibilities
- PI and research team qualifications
- Study objectives, eligibility criteria, recruitment, and procedures
- Space requirements, availability of a secure area to store investigational drug or devices, availability of required equipment
- Lab manual, specimen processing, and shipping
- Regulations and Good Clinical Practice (GCP) guidelines, informed consent requirements, Institutional Review Board obligations, adverse event reporting, drug accountability, source documentation, and records retention (regulatory documents and study file organization)
- Data forms review (Case Report Forms, or CRFs), including electronic data entry
If it is a large multi-site trial, a sponsor/Clinical Research Organization may choose to hold an Investigator Meeting in lieu of conducting site initiation visits. In this case, the meeting is held sometime after the Site Qualification Visit (link to Site Qualification Visit ) and prior to recruiting participants. The Investigator Meeting is usually only attended by the PI. However, if the PI is unable to attend, the sponsor/Clinical Research Organization may allow a co-investigator or research coordinator to attend. Travel to this meeting is coordinated and paid for by the sponsor/Clinical Research Organization.
Access to UW Electronic Medical Records
To use the University of Washington’s electronic medical record systems (ORCA and Epic) to identify eligible patients or capture clinical data about participants, you will need to request ORCA and Epic access for necessary research staff from User Access Administration .
If you have questions about ORCA access privileges or how to complete the form, contact: [email protected] .
If you access PHI for research purposes under an Institutional Review Board-approved HIPAA waiver, you must record and submit records of the dates and purposes of these disclosures to UW Medicine Compliance via the UW Medicine Disclosure Accounting online database.
UW MEDICINE COMPLIANCE (206) 543-3098 | [email protected] Accounting of Disclosures Use & Disclosure of Protected Health Information for Research Accounting of Disclosures of Protected Health Information
Pre-Screening to Identify Participants
To identify possible subjects for research, you may need to access Epic or ORCA to look at healthcare records. Even if you are involved in the clinical care of the possible subjects, you will need Institutional Review Board approval of a Waiver of HIPAA Authorization (and if the UW is the Institutional Review Board reviewing the project, a Waiver of Informed Consent and Confidentiality Agreement as well).
Members of your research team may need to complete Epic Training, which is described in the “Training/Credentialing” section below.

Clinical Research Billing for Research Procedures
There are complex federal and private payer rules that govern the conditions under which clinical services, items and tests associated with a research study can be billed to study sponsors, study subjects and/or their insurers. Research teams are required to use Epic to accurately bill for research procedures. Accurate research billing depends on planning and collaboration between the research team and a wide variety of individuals and offices before, during and after the study is initiated.
EPIC REVENUE CYCLE OPERATIONS EDUCATION [email protected] Research Billing Compliance Policies Research Participant Association in Epic Epic Billing Tools
Laboratory Medicine and Research Testing Services
If results of testing performed by University of Washington Lab Medicine will be part of data capture, you must maintain copies of lab normal ranges along with CLIA and CAP certifications.
Licenses & Accreditation
Lab Medicine is also the home department for Research Testing Services, which coordinates and provides research-related phlebotomy, CLIA-licensed testing, research-only testing, processing, and limited specimen storage.
UW LABORATORY MEDICINE Research Testing Service (206) 616‑8979 | [email protected]
Research Instrument Validation and Calibration
Scientific Instruments supports more than 18,000 pieces of patient care, laboratory, and research equipment spread across the greater Seattle area including UW Medical Center, Harborview Medical Center, Northwest Hospital & Medical Center, Seattle Cancer Care Alliance, UW Physician’s Neighborhood Clinics and a variety of other University, state, federal and other publicly funded agencies. Their records can be used to document compliance with TJC, CAP, CLEA, AABB, Food and Drug Administration, CMS, or other accrediting agencies’ requirements for equipment maintenance.
UW HEALTH SCIENCES SCIENTIFIC INSTRUMENTS Machine Shop (206) 616-5074 | [email protected]
Arranging Compensation for Research Participants
Trials often offer compensation to research participants to encourage and appreciate the time and effort involved in participation. Payments or travel/parking reimbursements to research subjects must be approved by the IRB as part of the research activities. You may need to work with your department to identify specific procedures for compensation of research participants.
UW HUMAN SUBJECTS DIVISION (206) 543-0098 | [email protected]
UW PROCUREMENT SERVICES (206) 543-4500 | [email protected]
UW TRANSPORTATION SERVICES 206-221-3701 | [email protected]
Register Study on Participate in Research
Researchers can post their trials to www.ParticipateInResearch.org , and potential volunteers can search for studies that apply to them. This website was developed by the Institute of Translational Health Sciences in partnership with the UW School of Medicine’s Office of Research and Graduate Education to connect research teams with members of the community.
Set Up the Study Binder
Regulatory binders help research teams organize their files, maintain regulatory compliance, and adhere to Good Clinical Practice (GCP) standards for record keeping practices for research involving human subjects. Most sponsors/Clinical Research Organizations will provide the organizational forms and supplies they require you to maintain throughout the trial.
Helpful background can be found in Section 8, “Essential Documents for the Conduct of a Clinical Trial,” of the Food and Drug Administration’s guidance document E6, Good Clinical Practice: Consolidated Guidance.
Do a Walk Through
To make sure you are prepared to conduct the trial, do a walk-through of the research procedures before you schedule the first visit:
- Confirm pre-screening steps in Epic and ORCA
- Create visit packets that contain your recruitment, consent, and data collection resources you will use when approaching participants
- Role play a recruitment conversation using the recruitment script
- Pretend to schedule a study visit
- Role play an informed consent discussion
- Walk from the place where you’ll meet the participant to the visit location(s)
- Make sure you have everything you need at the visit location(s): lab kits, MD orders, pharmacy communication, lab requisition slips, data collection forms, laptop to access eCRFs/regulatory docs, equipment calibrated and in working order, mailing/shipping containers
- Review data collection forms (CRFs) and confirm access to electronic data entry system
Training/Credentialing
Human subjects protections training.
There is no institutional-wide requirement for human subjects training at the University of Washington. However, many sponsors, funding agencies, UW departments, and collaborating institutions require that all members of the research team have completed a standard training course on the protecting human subjects in research. The Collaborative Institutional Training Initiative (CITI) web-based training meets the requirements of most industry sponsors. To take a course on the CITI website, you must register for an account and then affiliate yourself with the UW.
UW HUMAN SUBJECTS DIVISION TRAINING (206) 543-0098 | [email protected] Human Subjects Division website
Clinical Trial Policy Training
University of Washington’s Clinical Research Budget & Billing (CRBB) support office provides Clinical Trial Policy Training to ensure that all UW Medicine faculty have a uniform knowledge of the regulations governing clinical research and that they understand the internal processes that have been implemented in order to maintain compliance in clinical research billing.
Your research support staff may also need to complete the CRBB modules within the UW Medicine Clinical Research Staff Training Program (CRB1, CRB2, CRB3) .
HIPAA Training
UW employees who are involved with research conducted with UW Medicine facilities must complete “HIPAA Online Training” and sign the “UW Medicine Privacy, Confidentiality and Information Security Agreement” within the first 30 days of the individual’s first day as a member of the research team.
UW MEDICINE COMPLIANCE (206) 543-3098 | [email protected] HIPAA Privacy and Information Security Training
Epic Training
For trials that utilize UW Medicine clinical facilities, investigators (or their designees) are required to use Epic scheduling software to enter research subject enrollment status information and to forward study-related admission notifications to the UW Clinical Research Budget and Billing office. To do this work, members of the research team need to complete Epic training and obtain access to Epic.
Register for the Epic classroom training course, RES110: Epic Research Participant Enrollment.
EPIC REVENUE CYCLE OPERATIONS EDUCATION [email protected] UW Medicine Account Activation Request Form
Bloodborne Pathogens Training
Principal investigators are responsible for assessing research activities to determine if members of the research team have a potential for exposure to human blood and its components, human tissue, all human cell lines, human source materials, as well as medications derived from blood (e.g., immune globulins, albumin) and other potentially infectious materials. If your research activities involve human blood and its components, you and your research team are required to comply with the UW’s Bloodborne Pathogens Program, which includes a training requirement.
UW ENVIRONMENTAL HEALTH AND SAFETY Training Administration (206) 543-7201 | [email protected] Bloodborne Pathogens Program
Radiation Safety Training
Members of your research team may need to complete radiation safety training or review “Radiation Safety Training for Ancillary Personnel.”
UW ENVIRONMENTAL HEALTH AND SAFETY Training Administration (206) 543-7201 | [email protected] Radiation Safety Training for Ancillary Personnel Radiation Safety Training
Shipping Biohazards Certification
If your research team will package and ship specimens via land, air, or sea, all team members must be trained and certified to ship hazardous materials. There are prescriptive requirements for packaging and labeling of hazardous materials and for the associated documentation used in the event of an emergency. There are fines for lack of certification and improper packaging and, worse, a chance for loss of life and property.
UW ENVIRONMENTAL HEALTH AND SAFETY Training Administration (206) 543-7201 | [email protected] Shipping Hazardous Materials
UW Medical Center Credentialing
The UW Medical Center credentialing process ensures that individuals other than physicians, Nurse Practitioners, and Physician’s Assistants who interact with patients at UW Medical Center are competent to practice in their role and have current immunizations to ensure the safety of the patients. Members of your research team interacting with UW Medical Center patients must have current credentialing.
UW MEDICAL CENTER CREDENTIALING [email protected] Credentialing
Site Visits
- First Online: 17 May 2017
Cite this chapter

- Kamal M. F. Itani 3
1354 Accesses
Site visits are planned activities of large multi-institutional prospective clinical trials. Although, these visits are planned differently for different trials depending on complexity and number of participating sites, it is not uncommon to have a pre-qualification site visit, an initiation site visit, monitoring site visits, and close-out site visits. Unplanned or planned site visits can be conducted by the FDA or sponsor for trials involving new drugs or medical devices. These site visits can also be the result of monitoring findings or for quality assurance purposes. For research funded by the Department of Health and Human Services, the Office for Human Research Protections can also conduct site visits for cause or not for cause.
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
- Get 10 units per month
- Download Article/Chapter or Ebook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Compact, lightweight edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
- Durable hardcover edition
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
Similar content being viewed by others

Responsibilities and Management of the Clinical Coordinating Center

Centers Participating in Multicenter Trials
Neumayer L, Giobie-Hurder A, Jonason O, Fitzgibbons R, Dunlop D, Gibbs J, Reda D, Henderson W, The VA Cooperative Studies Investigators. Open versus laparoscopic mesh repair of inguinal hernia. N Engl J Med. 2004;350:1819–27.
Article CAS PubMed Google Scholar
Download references
Author information
Authors and affiliations.
Department of Surgery, VA Boston Health Care System/Boston University and Harvard Medical School, VABHCS (112A), 1400 VFW Parkway, West Roxbury, MA, 02132, USA
Kamal M. F. Itani
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Kamal M. F. Itani .
Editor information
Editors and affiliations.
Department of Surgery, Boston VA Healthcare System Department of Surgery, West Roxbury, Massachusetts, USA
Kamal M.F. Itani
Depatment of Veteran Affairs, Cooperative Studies Program Coordinating Center, Hines VA Hospital, Hines, Illinois, USA
Domenic J. Reda
Rights and permissions
Reprints and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Itani, K.M.F. (2017). Site Visits. In: Itani, K., Reda, D. (eds) Clinical Trials Design in Operative and Non Operative Invasive Procedures. Springer, Cham. https://doi.org/10.1007/978-3-319-53877-8_38
Download citation
DOI : https://doi.org/10.1007/978-3-319-53877-8_38
Published : 17 May 2017
Publisher Name : Springer, Cham
Print ISBN : 978-3-319-53876-1
Online ISBN : 978-3-319-53877-8
eBook Packages : Medicine Medicine (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
- Conference Coverage
- CRO/Sponsor
- 2023 Salary Survey

- Publications
- Conferences
Selecting Study-Appropriate Clinical Sites in 3 Steps
Applied Clinical Trials
These three steps should take place in order to ensure an optimal site selection.

A compilation of recent notable news developments that pertain to the clinical trials industry.
Initial Design Considerations for Immuno-Oncology Trials
Perspectives and insights regarding regulatory considerations when planning and conducting immunotherapy studies.

Regulatory Collaborations Aim to Speed Access to New Vaccines and Drugs
The increasing need for treatments to slow the global spread of COVID-19 has prompted greater cooperation between drug regulatory authorities around the world.
How Digital Technology and Remote Assessment Strategies Can Aid Clinical Trial Research
While there's been hopeful news on treatments and vaccines, sponsors should plan to discuss necessary strategies and contingencies at the outset of new studies or re-opening of halted studies during the COVID-19 pandemic.
Regulatory Considerations Surrounding Human Challenge Studies with the SARS-CoV-2 Virus
A first vaccine against this coronavirus could still take some time to develop, but mRNA vaccine platforms could offer an early breakthrough.
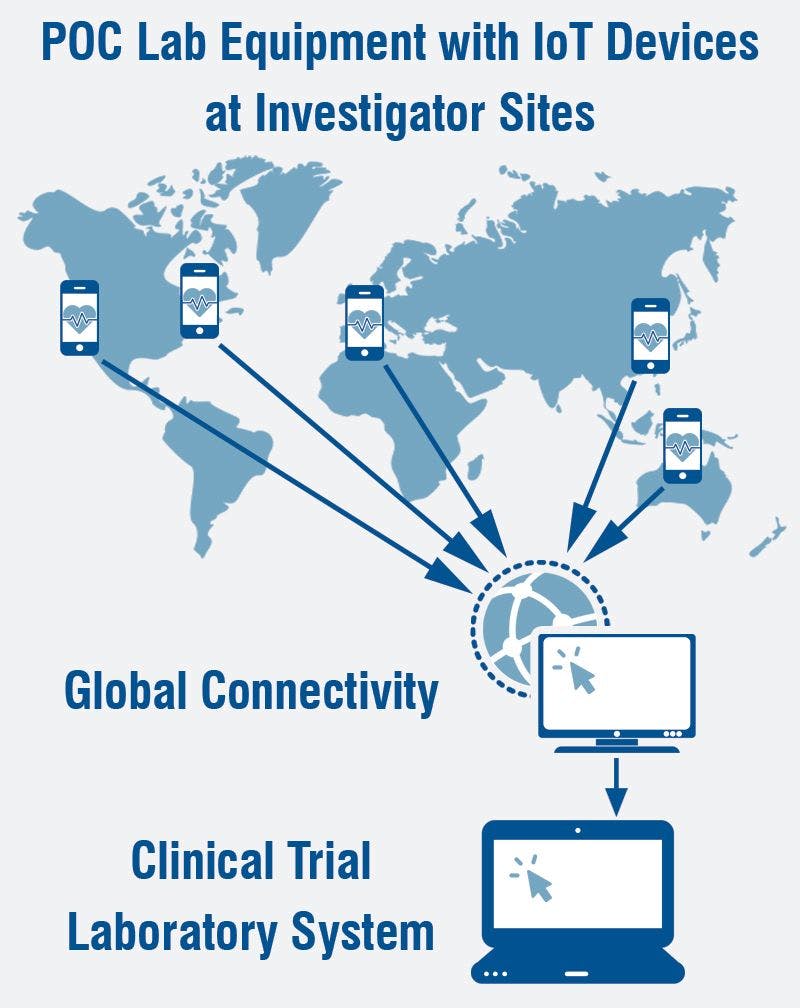
Will Point of Care Laboratory Devices Replace Brick and Mortar Labs in Clinical Trials?
Over the past few years, there has been significant growth in the use of more complex point of care laboratory devices.
2 Commerce Drive Cranbury, NJ 08512
609-716-7777

Site Qualification Visits and Site Initiation Visits
Thank you to Patient Recruitment Centre: Leicester for providing this content Version 1 - March 2023
Download .pdf version of Checklist here
Site Qualificatio n Visit Checklist
The purpose of a SQV is to assess whether it is feasible for a site to run a study from the sponsor perspective. You will still need an internal feasibility assessment to discuss the study in much more detail, in particular recruitment strategies and targets.
Consider making a video tour of your facilities since this reduces staff burden and provides an overview of site. It also showcases your research department and can be used during the EOI process. You can find examples of this here .
Book a room and establish if in person or virtual.
Be prepared to give a tour of your facility including relevant departments e.g. pharmacy.
Ensure you have display screen equipment for SQV slides. If your organisation does not allow encrypted or external devices, request that the slides are sent in advance.
Invite appropriate people from support departments e.g. pharmacy or radiology, or consider if they will have a separate meeting with the sponsor.
Make sure you can accommodate the number of attendees, internally and from the sponsor and/or the CRO and allow for additional guests.
Preparation Needed
Ensure your department is clean and tidy and be aware of confidentiality with departmental documents.
Review material provided e.g. protocol synopsis, training slides and compile questions.
Check you have equipment required e.g. fridge, freezer, centrifuge, and space resource. If not, make a list of what is required.
Collate any information about previous sponsor audits or site inspection outcomes.
During the Meeting
Ask the following questions:
What is the status of the study?
What are the study timelines?
What is the expected target?
What is the recruitment period?
Number of UK sites?
Is the protocol finalised? Can amendments be suggested by PI and site staff?
If the study is already open what have the challenges been?
What is the screen failure rate?
Identify any equipment that the sponsor will need to provide or fund and who will order them. Ensure this is discussed and clear at an early stage. Do not wait until SIV when the contract will likely have been finalised.
Review recruitment strategy.
Will they allow PIC sites?
What support do you need from the sponsor e.g. what advertising materials are provided? Is there the opportunity to suggest alterations?
Ask when the sponsor will inform the site if they have been selected or not.
After the Meeting
Follow up with any actions.
Be prepared to provide documentation such as GCP certificates, CV’s, calibration certificates, FDF, and a contact list of staff.
If you are not selected as a site, remember to ask for feedback.
Site Initiation Visit Checklist
Th e sponsor run the SIV, however the lead site staff can utilise this opportunity to ask any remaining queries regarding the protocol and identify any outstanding requirements.
Ensure you have display screen equipment for SIV slides. If your organisation does not allow encrypted or external devices, request that the slides are sent in advance.
Invite all staff who will be working on the study. If they are not available they can review the slides after the event. Best practice would be to book SIV when all key site staff are available. The PI may only be needed for part of the meeting.
Invite appropriate people from support departments e.g. pharmacy or radiology and consider if they will have a separate meeting with the sponsor.
Make sure you can accommodate the number of attendees internally and from the sponsor/CRO. Be prepared for additional external staff to attend.
Some SIVs can last the full working day. Ensure you are clear on how long the meeting is meant to be and ensure you are aware of lunch arrangements i.e. is the sponsor providing or funding.
Prepare any questions you have about the study beforehand.
Review whether everything is in place for starting the study - IMP, lab kits, system accesses, all documents including site files, equipment e.g. ECG, medical devices. This may not be the case for some studies with expedited set up.
Ensure you know the protocol.
Ensure someone is in attendance that knows the contract, in case any activities are discussed that are not included in the contract.
Complete the SIV attendance log or send a list of attendees to the CRA.
Circulate the delegation log if not already completed.
Raise any queries about missing equipment, documents etc.
Review inclusion and exclusion criteria.
Source document review - Agree what documents are source and what electronic systems the monitors will need access to.
Decide who will be reviewing the safety reports.
Record the SIV training on the finance tracker so it can be invoiced.
Put a plan in place for screening the first participant.
Review the participant recruitment pathway.
Consider a dummy run especially if numerous support departments are involved.
Request any amendments to the contract that are identified.
Make worksheets if not already prepared at this point.
Conducting Successful Site Qualification Visits using Virtual Solutions
August 18, 2020
On-site facility evaluations have historically provided us with the insight necessary to determine site performance and viability for our clinical trials. With decentralized trials as a mainstay of the current clinical research landscape, it’s imperative to leverage the virtual resources available to cultivate those same meaningful evaluations and assessments, safeguarding patient safety and data integrity. We were able to achieve such success when we completed 12 virtual, customized, site feasibility assessments of facilities located throughout the U.S. for a respiratory study. Given the current resurgence of COVID-19, the safety of site staff and our study team was paramount. To minimize the risk to the sites and our CRAs, we elected to conduct the site qualification visits virtually, which required creating hybrid approaches to utilizing our virtual platforms.
Pre-planning
Advance preparations were critical when it came to planning our virtual Site Qualification Visits (SQV). Based on our prior decentralized trial experience, we were able to quickly assess the data we needed from our virtual visits to make the most confident decisions selecting our sites. After reviewing each site’s feasibility questionnaire, we emailed the site asking them to provide their scheduling availability over the next few weeks. In addition, we realized the value of having all the stakeholders together in the meeting and requested that the following key site personnel attend the visit, which we expected to take 3-4 hours.
- Principal Investigator (required, approximately 1 hour)
- Research Coordinator(s) (required, entire call)
- Pharmacist (required, 30 minutes)
- Sub-Investigators (optional)
- Regulatory staff member (optional)
- Contracts/budgets Manager (optional)
We were able to manage expectations by noting the amount of time each person would be needed on the call and we offered flexible scheduling options to accommodate staff that were unavailable on the primary visit date/time.
Virtual Platform Solutions
During the remote SQVs, we wanted to tour the facilities and equipment, including the pharmacy, and made sure that the sites had the ability to conduct a video tour of the noted facilities during the call. Our CRA confirmed access to alternative video-conference technology with the site that we could use as “back-up” if platforms such as WebEx or FaceTime didn’t work or weren’t available. Some platforms may compromise organizational security and confirming which technologies are available and/or accessible per institutional IT policies are key to ensuring a successful virtual visit.
The assigned CRA set up a WebEx session with web camera capabilities as one method of conducting the virtual tour. One of the sites had pre-recorded a video tour of their facilities and had the link accessible to preview via YouTube, which the CRA was able to view as an additional resource. The live tour was conducted during the SQV to confirm that all study specific requirements were met. Clearly, sites are adjusting to this COVID-world and are proactively finding ways to address the standard request.
The main presentations were conducted via WebEx and then the Site Coordinator/CRA had the option to switch to FaceTime for the tour, which proved to be a more convenient option using a smaller device to conduct the tour. Of note, there are other virtual platforms, such as WebEx, that have robust phone applications, which may be preferable to carrying a laptop around a facility. The transition from WebEx to FaceTime took some extra time, but once that happened, the CRA communicated this change to site personnel for subsequent visits, so that they were prepared and timelines weren’t impacted.
Of the 12 SQVs we completed, we selected 8 viable sites and 2 alternative sites based upon the nuanced criteria we collected during the tour. We leveraged our past experience to adapt our preparations for the SQVs. The virtual platform strategies we employed significantly shortened the SQV time period within the study start up timelines, reduced travel costs, created user friendly assessment options and flexibility, and above all, protected all staff from any COVID-related risks.
Hybrid virtual applications in clinical trial processes can positively impact the course and integrity of the trial. To realize the advantages and benefits of a well-executed virtual clinical development program, it’s critical to partner with a CRO with experience planning and implementing successful decentralized solutions.

- Why 1ˢᵗ Credentialing? + –
- Leadership + –
- Testimonials + –
- Client List + –
- Applications + –
- Maintenance + –
- 1stCred + –
Who We Serve
- Behavioral and Mental Health Facilities + –
- Hospitals + –
- Cardiology + –
- Eye Care + –
- Orthopedics + –
- Podiatry + –
- Multispecialty Groups + –
- Partnerships + –
- Telemedicine + –
- Our Blog + –
- Newsletters + –
- Videos + –
- Contact + –
CMS Site Visits – How Would Your Practice Do?
The Centers for Medicare & Medicaid Services (CMS) requires an onsite review of Medicare enrolled providers and suppliers under certain conditions.
The reviews are required to ensure that these healthcare providers actually exist and that they meet the requirements to provide services to Medicare beneficiaries. The onsite visits are integral parts of the enrollment validation process. Generally, the visits are required for moderate to high-risk providers during the initial enrollment, revalidation and when a new location is added.
You can read about the basic requirements for the onsite visits in our earlier blog .
Would Your Practice Pass the Onsite Review?
Even if you are fully compliant with the enrollment rules, you are still at risk of revocation of billing practices if you fail the onsite visit. For example, the site inspector may not be able to locate your business if the outside signage is missing, your enrolled address is wrong or if your practice is not open at the time of the visit.
Do a pre-visit audit yourself to avoid billing revocation because of a problem at an onsite visit:
- Check that CMS has complete and current information for each of your locations.
- Make sure that you have a process in place to report any changes including address, telephone number and hours of operation.
- Take a look at your signage for accuracy. Make sure hours of operation are clearly posted.
- Add any necessary information to find your practice if you are located in another facility like a hospital or nursing home, or if your building prohibits signage.
- Site verification visits are made between 9:00am and 5:00pm. If your office is not regularly open during those hours, make sure your enrollment information includes your hours of operation.
Be proactive in this process! If CMS determines that your practice is not operational, you risk revocation of billing privileges.
Let Us Manage All Your Payer Enrollment Services
If you require medical credentialing and payer enrollment needs for your practice or medical facility, please contact 1st Assistant. Our experienced and dedicated specialists will provide all credentialing and enrollment services quickly and will monitor your account for ongoing updates and re-attestations. Heidi Henderson , our company owner and President, is eager to meet you and discuss your payer enrollment needs. Please call us at 512.201.2668 or contact us via the website .

Categories: Knowledge Center , Medicare and Medicaid
Free Consultation
Thank you for your interest in our services. Let’s schedule a call to talk more about your project and how 1ˢᵗ Credentialing can help. Click here to book an appointment.
Book an appointment
Schedule your free consultation
Our credentialing experts are here to help you assess exactly which solutions you need to put you on the right track. 1ˢᵗ Credentialing includes payor enrollment for all insurance networks. Don’t wait another minute, contact our team today!
Call us at (512) 201-2668 or email us at [email protected]
Stay In the Know
Find out how we can save your time and money by scheduling your free consultation.
Schedule with Calendly
*Your information may be shared with 1st Credentialing’s trusted partners.

Site Initiation Visits: Starting Your Trial On Track

Presenting The Protocol The CRA’s task is not only to present the protocol, but to engage the team. The doctors, nurses and pharmacists involved in the study are busy, and making them sit through hundreds of presentation slides may not give the trial the best start. Instead, the CRAs give the team the chance to ask questions, in an interactive session. They answer any questions the PI and other staff have about the study, and address any issues before the trial starts. The presentation might cover the design of the trial, the drug or treatment being tested, and any relevant background information about the therapeutic area, always keeping the audience in mind. Details like eligibility criteria for enrolment, how to store and administer the drug, and quality management points are also included.
The Trial Walk-Through In a meeting with everyone involved, the CRA will lead the group in a walk-through of the study, often from the perspective of a participant. By looking at the process from enrollment to trial end, the CRA can ensure the whole team understands what lies ahead in the trial. They can also identify any gaps in knowledge and spot potential problems before they arise.
Checking Documentation And Equipment The SIV includes some logistical and physical checks. The CRA checks that the drug is on-site, available and correctly stored. They ensure the team has the necessary equipment, such as lab kits or ECG machines, and that those who need access have it. The CRA checks the relevant documentation has been completed, such as regulatory documents, informed consent documents. Finally, they ensure the relevant team members have access to the systems and portals they will use during the trial.
Why Experience Matters
While every study is different, a CRA applies the same skills to make each SIV a success. With experience across a range of trials in different therapeutic areas, a CRA can take the most effective approach to their presentation of the protocol, answer questions about the trial with confidence in their knowledge, and cover all bases to get the trial started in a positive direction.
At Siron Clinical, our CRAs have at least 15 years’ experience in setting up and running clinical trials – including site initiation visits. Find out more about how we can support you .

STARTING A CLINICAL TRIAL IN THE EU?
Download our RFI to learn more. You'll learn how you can leverage our experience to help you with your clinical trials.
Check your email for the ebook!
Submit a comment cancel reply.
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Submit Comment
Debate takeaways: Biden confirms some voter fears as Trump leans into grievances
ATLANTA — President Joe Biden failed to mitigate his biggest liability in his re-election bid at the CNN debate Thursday, while former President Donald Trump doubled down on his grievances and skipped past opportunities to cover his own vulnerabilities.
Biden's biggest weakness — voter concerns about his age and sharpness, according to polls — was on display throughout the more than 90-minute debate as he struggled through answers and failed to deliver the energetic performance allies believe he needed. And Trump had no new answers for voters about the issues on which he's weakest, including his felony conviction, his role in overturning Roe v. Wade and his actions on Jan. 6, 2021.
The first showdown between Biden, 81, and Trump, 78, in 2024 comes early in the election year, giving voters an opportunity to see the president and his challenger side-by-side. Here's what they saw — and what it means for the campaign.
Biden struggles out of the gate
The first presidential debate is often rough for incumbents . It was particularly rough for the 81-year-old Biden out of the gate.
When he began Thursday night, his voice was hoarse, his throat didn't sound clear, and he started out speaking softly and struggling through some of his responses. His voice cracked throughout the debate. Biden's campaign later s a id he ha d a cold .
In one particularly notable gaffe, Biden stumbled through a response to an early question about rising costs, ending with: “We finally beat Medicare.” The Trump campaign mockingly highlighted the clip on his social media platform, Truth Social.
During a clash over immigration, Biden stammered through some words while expressing his support for tougher border laws, to which Trump responded: “I really don’t know what he said at the end of that sentence. I don’t think he knows what he said, either.” Trump repeatedly accused Biden of implementing a weak border policy that he said has worsened crime in the U.S.

Even Biden’s closest allies said it was a bad night for him. “It was a really disappointing debate performance from Joe Biden,” former Biden communications director Kate Bedingfield said on CNN.
Biden did find his footing when he was speaking about foreign policy, protecting NATO and standing up to Russia. He also tore into Trump over Jan. 6 and blasted him for saying there were “very fine people” on both sides when neo-Nazis marched in Charlottesville, Virginia, in 2017. Later, when he was asked whether he has a vision to protect Social Security, Biden said: "Yes, make the very wealthy begin to pay their fair share."
Toward the end, Biden defended running for re-election at his age when the moderators asked him to address voter concerns. “This guy’s three years younger and a lot less competent,” Biden said.
Trump descends into grievances
Trump's long-standing tendency to retreat into airing personal grievances, often at the expense of defending himself or his record, reared up throughout the evening.
When CNN’s Jake Tapper and Dana Bash asked him to address voter concerns about his actions on Jan. 6, when pro-Trump rioters stormed the Capitol, Trump took the opportunity to blast the House Jan. 6 “un-select committee” and its “two horrible Republicans.” When Biden brought up Trump’s alleged affair with Stormy Daniels, Trump took the bait: “I didn’t have sex with a porn star, No. 1,” he said.
Trump was also evasive when he was asked repeatedly whether he will accept the 2024 election result.
“If it’s a fair and legal and good election, absolutely,” Trump said, repeating his false claim that the 2020 election result was illegitimate: “The fraud and everything else was ridiculous.” In response, Biden mocked him. “You’re a whiner,” he said. “You can’t stand a loss; something snapped in you when you lost last time.”
Ten minutes into the debate, Trump launched a groundless claim that Biden is weaponizing American justice against him. He called it "a system that was rigged and disgusting," as he faces criminal charges across multiple jurisdictions brought by independent prosecutors. Toward the end, Trump made the same allusion again: “He indicted me because I was his opponent.”
Trump also brought up the Russia investigation — “Russia, Russia, Russia,” as he called it — as well as Biden’s son Hunter Biden’s laptop computer.
And Trump leaned into his promise of retribution if he wins the presidency this fall. “He could be a convicted felon as soon as he gets out of office,” Trump said of Biden. Trump also said, "My retribution is my success."
Clashes over abortion, taxes and more define the policy stakes
Trump embraced his role in appointing the three "great" Supreme Court justices who voted to overturn Roe v. Wade while repeatedly — and falsely — claiming that there was complete consensus about ending the federal right and returning abortion policy to legislators in 2022.
“Everybody wanted to get it back to the states,” Trump said — polls show voters supported Roe v. Wade by large margins. “This is something everybody wanted.” He added that “every legal scholar wanted it that way” — many of them disagreed.
And Trump embraced an argument popular among social conservatives, arguing that Democrats were the real radicals on abortion by refusing to support federal restrictions. After Trump claimed that Biden supports abortions "after birth," Biden retorted by saying he favors only restoration of Roe v. Wade, which allowed for some limitations. “We are not for late-term abortion. Period. Period. Period,” Biden said.
Trump focused significant attention on the border and immigration, criticizing rising migration during Biden's term.
And he also called for extending his administration's 2017 tax cuts, which expire at the end of 2025. Biden promised, repeatedly, to raise taxes on the rich, saying Trump “rewarded the wealthy — he had the largest tax cut in American history.”
Biden’s prepared zingers
Biden’s sharpest moments came when he delivered what appeared to be prepared one-liners at Trump, often calling him a liar or a felon and frequently dismissing his claims with a dismissive grin.
“The only person on this stage who’s a convicted felon is the man I’m looking at right now,” Biden said, drawing a nod from Trump.
When Trump attacked his record on immigration, Biden said, “Once again, he’s exaggerating, he’s lying.” His other lines included: “Every single thing he said is a lie. Every single one.” And: “I’ve never heard so much malarkey in my whole life.”
At one moment, Biden attacked Trump for calling service members “losers” and “suckers,” according to reporting by The Atlantic . “My son was not a loser. He was not a sucker. You’re the sucker. You’re the loser,” Biden said. (Trump responded by questioning the magazine’s credibility.)
When Trump said Biden has “become like a Palestinian,” Biden replied: “I’ve never heard so much foolishness.”
And Trump brought some prepared lines of his own, including one at the end, when he argued the U.S. is “a failing nation, but it’s not going to be failing anymore — we’re going to make it great again.”
“We’re living in hell,” Trump said. “The whole country is exploding because of you.”
Sahil Kapur is a senior national political reporter for NBC News.

IMAGES
VIDEO
COMMENTS
Check that your facility has met the necessary security requirements in regard to drug storage, data security and access. Site Qualification visits are an essential component of the clinical trials site selection process. This visit allows both you and the trial's Sponsor to learn more about each other and ascertain if the study is a good fit.
Pre-study visits (site selection visits or site qualification visits (SQVs)) are conducted to determine if the investigator and clinical site have the capability to conduct the study. ... When the research study has been completed at a site, a close-out visit occurs. This type of visit can take the form of an on-site visit or, in some cases, be ...
Here are some best practices for conducting clinical site qualification visits: Prepare in advance. Preparation is critical to the success of a site qualification visit. Before the visit, review the study protocol, site-specific documents, and any other relevant study materials to understand the site's capabilities and needs.
Effective Date: 01-JUL-2017 Site Qualification Visit Page 1 of 4 . SOP-05: Site Qualification Visit . 1. Objective To ensure that the Principal Investigator (PI) and all research team members assisting in the conduct of clinical research are informed about their obligations and responsibilities as they pertain to Good Clinical Practices
CMS site visits take place during posted hours or on Monday through Friday between 9:00 a.m. and 5:00 p.m. If the auditor arrives during the hours shown on your sign and no one is there he can record your business as not operational. The same is true if the auditor is unable to locate your business because the sign is not in plain view or doesn ...
With remote clinical trial monitoring, 75% of CRAs visit 2-5 sites per week, increasing their efficiency for IMVs by 2-5x. CRAs can use a Site Enablement Platform to remotely: Review Drug Accountability Forms, Case Report Forms (CRFs), and logs. Access dashboards that show how the study is progressing at each site.
Sponsors and CROs may lose trust in the site and the staff. 3 Steps for a Successful Monitoring Visit. With proper communication and a simple 3 step process, preparing for a monitoring visit does not have to be overly time-consuming. Plus, spending time in advance will be time saved through an efficient, organized visit! Plan the visit.
Definition. Site qualification is the process by which the study sponsor and/or clinical research organization determine whether the investigator and the clinical site have the resources and capabilities necessary to conduct the study. The sponsor may require completion of a feasibility questionnaire. The Office of Clinical Trials can assist in ...
Site Qualification Visit (SQV) Engage the client in evaluating the benefit of having a qualification visit. SQVs can be performed remotely with the support of other factors and documentation. If need be, waiving the formal visit may be an option if procedures and policies allow. Items to consider:
If it is a large multi-site trial, a sponsor/Clinical Research Organization may choose to hold an Investigator Meeting in lieu of conducting site initiation visits. In this case, the meeting is held sometime after the Site Qualification Visit (link to Site Qualification Visit) and prior to recruiting participants. The Investigator Meeting is ...
A Site Initiation Visit (SIV) or Study Start-Up is an organized meeting to discuss the new protocol before the research project is ready to screen and enroll potential patients. It also serves as training for the protocol of interest. All members on the study (everyone listed on the Delegation Log and IRB
Site visits are planned activities of large multi-institutional prospective clinical trials. Although, these visits are planned differently for different trials depending on complexity and number of participating sites, it is not uncommon to have a pre-qualification site visit, an initiation site visit, monitoring site visits, and close-out site visits.
Step 1: Define Site Requirements and Selection Criteria. One of the most cited inefficiencies in the industry regarding site selection is the lack of information about the protocol when starting the process. 3. The first step to selecting appropriate sites for a study is to identify key site criteria from the study design as this data provides ...
Site Qualification Visits and Site Initiation Visits. Site Qualification Visit Checklist. The purpose of a SQV is to assess whether it is feasible for a site to run a study from the sponsor perspective. You will still need an internal feasibility assessment to discuss the study in much more detail, in particular recruitment strategies and targets.
Conducting Successful Site Qualification Visits using Virtual Solutions. On-site facility evaluations have historically provided us with the insight necessary to determine site performance and viability for our clinical trials. With decentralized trials as a mainstay of the current clinical research landscape, it's imperative to leverage the ...
Definition. Site initiation is the final step in the study set-up process. Site initiation occurs prior to site activation. Typically, the sponsor or clinical research organization will conduct a site initiation visit after the Institutional Review Board approves the clinical research study at that site and the clinical research study agreement ...
Site verification visits are made between 9:00am and 5:00pm. If your office is not regularly open during those hours, make sure your enrollment information includes your hours of operation. Be proactive in this process! If CMS determines that your practice is not operational, you risk revocation of billing privileges.
Request medical records to be reviewed. Arrange for a quiet room. Inform pharmacy of visit and schedule appointment. Make sure PI and AIs will be available for monitoring date. If multiple monitoring visits occur simultaneously, make sure each sponsor has a separate room to ensure privacy and confidentiality.
What. Prior to study enrollment, the study monitor on behalf of the sponsor will conduct a Site Initiation Visit (SIV) to provide the principal investigator and the study team training on the protocol, procedures, processes and monitoring plan. The monitor will also review the responsibilities of the investigator ( 21 CFR 312 Subpart D ).
The study initiation visit is a meeting arranged and conducted by Georgia CORE and the sponsor, if applicable, to complete the final orientation of the study personnel to the study procedures and GCP requirements. It occurs after the pre-study site visit when all study arrangements have been concluded or are almost complete, and the study is ...
by Siron Clinical | Mar 31, 2022. To get the best start with a clinical trial, we carry out a site initiation visit (SIV). In this post, we look at what that entails and how we ensure it sets the trial off in a positive direction. When setting up a clinical trial, there are many steps to take, both on the approval side and the logistics side.
Effective Date: 01-OCT-2019 Site Qualification Visit Page 1 of 4 . SOP-05: Site Qualification Visit . 1. Objective To ensure that the Principal Investigator (PI) and all research team members assisting in the conduct of clinical research are informed about their obligations and responsibilities as they pertain to Good Clinical Practices
The result does not take away from an incredible weekend for the high schooler after he set — and then bested — the world record for under-18 runners that stood for 42 years.
Biden's halting performance overshadowed a debate in which Trump declined opportunities to assuage voter concerns about the Jan. 6 Capitol riot and his legal troubles.