
- There are no more items in your cart
- Total $0.00
- News & Tips
- Technical Information
- Instructions
- The Wolfgang Catalog - Truly Unique
- Case & Components
- Crankshaft Assembly
- Pistons + Heads
- Cams + Rockers
- Intake, Fuel & Filters
- Carburetors & Components
- Oil Systems
- Stock Exhaust
- Aftermarket Exhaust
- Gas Tanks & Components
- Heater Components
- Transmission
- Front Beam & Components
- Spindles & Components
- Bearings, Spacers, & Seals
- Sway Bars & Components
- Shocks & Components
- Super Beetle Only
- Aftermarket
- Drums/Rotors
- Wheel Cylinders
- Master Cylinders & Components
- Brake Lines & Components
- Miscellaneous Hardware
- Front Brake Kits
- Rear Brake Kits
- Miscellaneous Components
- Adapters & Spacers
- Lug Bolts, Nuts, & Studs
- Misc. (Beauty Rings, Knockoffs, and Hubcaps)
- Frame & Components
- Bumpers & Components
- Cables & Components
- Pedals & Components
- Shifters & Components
- Emergency Brake & Components
- Replacement Sheet Metal
- Engine Bay Components
- Front Hood Bay Components
- Front Trunk
- Door Seals & Components
- Windows, Seals, & Components

Safari Windows & Components
- Interior Parts
- Front Trunk Carpet Kits
- Carpet Kits
- Door Panels & Components
- Rear Well Carpet Kits
- Misc. Interior Upholstery Parts
- Seats, Seat Covers, & Components
- Sunroof Parts
- Convertible Parts
- Exterior Trim & Components
- Sending units
- Rear Lights
- Turn Signals
- Interior Lights
- Relays & Horns
- Wire Harness & Battery Cables
- Generators, Alternators, & Starters
- Engine Ignition Components
- Accessories
- Engine Mounts
- Overflow tank
- Stock Exhaust Up to 1971
- Stock Exhaust 1972-1974
- Stock Exhaust 1975-1978
- Stock Exhaust 1979
- Stock Exhaust 1980-1983
- Stock Exhaust Diesel
- Heater Assembly Up To 1971
- Heater Assembly 1972-74
- Heater Assembly 1975-78
- Heater Assembly 1979
- Heater Assembly 1980-83
- Camper Top Components
- Case + Components
- Intake, Fuel + Filters
- Carburetors + Components
- Gas Tanks + Components
- A-Arms & Components
- Sway Bar & Components
- Window, Seals, & Components
- Misc Interior Upholstery Parts
- Seats, Seat Covers & Components
- Sending Units
- Lug Bolts, Nuts & Studs
- Rear Well Carpet Kit
- Steering Brakes
- Adaptors & Spacers
- Mounting Tabs & Brackets
- Fiberglass Body Panels & Components
- Electrical Fittings
- Books & Manuals
- Fluids & Sealers
- Upholstery Colors
$0.00 - $1,239.00
Part #: N109051
Side Popout Window Frame Screws (3.5mmX6mm) Frame to Hinge
Screws for replacement pop out window frame to hinge
- Bus (Type 2): -67
Part #: N139681
Screws - Safari mounting screw
This is a list of the most common screws you might need..
- Bug (Type 1): 58-64
- Bus (Type 2): All
Part #: N185164
Side Popout Window Screws (4mmX10mm) For Latches to Body
Screws for replacement pop out window Latches to Body
Part #: 211837659
Safari Friction Washers, Each
This nylon washer goes on the wing bolt keeping the bolt from chewing up the slide on the safari window. Washers sold individually 8 are required for Front Safaris and 4 are required for Rear Safaris.
- Bus (Type 2): 55-67
Part #: N107101
Screws - Tin screw w/washer
- Bug (Type 1): All
- Type 3: All
- Race/Off-Road: All
Part #: N109052
Side Popout Window Frame Screws (3.5X5) To join frame halves together
Screws for replacement pop out window frames. These screws are for joining the two Popout frame halves together

There are 21 products.
Part #: 1847100CWG
Bug Safari - Chrome
This Bug Safari window is constructed as a dual-channel, mild-steel frame with a chrome finish that accepts 3/16" safety glass and an outer lip seal. This chrome options arrives pre-assembled
- Off-Road: 58-64
Part #: 1847100KWG
Bug Safari - Raw Steel
This Bug Safari window is constructed as a dual-channel, steel frame that accepts 3/16" safety glass and an outer lip seal. The basic "Assemble-At-Home". The raw steel option is for those who want to completely customize their window frame.
Part #: 1845101KFR
Raw Bug Safari Frame ONLY
- Bug (Type 1): 1958-64
Part #: 1845111AL
Bug Safari Glass to Frame Seal
Part #: 1845121A
Body to Frame Seal Bug Safari
Body to Frame Seal Bug Safari This seal is on the body of the car and goes between the body of the car and your new Safari Frame
Part #: 1845131AL
Bug Safari Frame to Body Seal
Bug Safari Frame to Body Seal This is the Lip Seal Style Seal that goes on the outer edge of your Safari Frame and mates to the Body of the vehicle
Part #: 1845101KWG
Bug Safari Glass ONLY
Part #: 1847101LASY
Hinge Assembly Bug Safari Left
Part #: 1847101RASY
Hinge Assembly Bug Safari Right
Part #: 211847516
Safari Latch - Factory safari's or new style WG safari's, handle points left.
This latch screws onto the safari window latching it closed.
Part #: 211847515
Safari Latch - Factory safari's or new style WG safari's, handle points right.
Part #: N109062
Safari Latch To Frame Screw OE Style Latch
Follow us on facebook.
Your cart is empty
It feels desperately alone.

- You are here:
- SPLIT WINDOW
- WINDOW RUBBER - GLASS
Safari Front Windshield Kit; Bus; 1955-1967; Stainless Steel
Polished stainless steel safari window kit.

- Create New Wish List

Email us at [email protected] and be sure to include the Item #. Contact Us
We recently launched our new site. Your feedback is appreciated. Email us at [email protected] and we'll be in touch. Contact Us
New Safari Window kit. Polished stainless steel
*Kit does not include wiper shafts or one eyed duck
Made in USA.
- 1955-1967 Type 2 Bus (all models)
Write a Review

- Our Experts Recommend

Item #211955231
Safari window wiper blade rest.

Item #211955407A
Wiper arm - buses thru 1967; each.

Item #211955225C
Safari wiper shaft; 1965-1967.

Item #211955425C
Bus wiper blade - single.

Item #WG2847100WH
Safari front windshield kit; bus; 1955-1967; off-white.

Item #WG2012900
Accessory safari antenna.

Item #N114805
Safari window wiper blade rest retaining screw, select your model type.
- Convertible
- Split Window

- Info about VWs
- Splitscreen
- T3 & T25
- Karmann Ghia
- T4 Transporter
- T5 Transporter
- T6 Transporter
Contact us 01322 33 50 50
- Login / Register
- including VAT
- excluding VAT
- Buggy and Trike
- Miscellaneous
- Gift Vouchers
- Manufacturers

- Exhaust and Heating
- Gearbox and Clutch
- Pedal and Handbrake
- Shifters and Linkage
- Body, Door and Roof
- Rubbers and Seals
- Repair Panels
- Shifter and Linkage
- Body Rubbers and Seals
- Fuel System
- Cooling System
- Exhaust System
- Transmission
- Body and Door
- Engine Parts
- Transmission Parts
- Steering Parts
- Suspension Parts
- Hubs and Wheel Bearings
- Interior Parts
- Body Panels
- Rubber Seals
- Electrical Parts
- Oils, Fluids, Lubricants and Paint
- Hoses and Fittings
- Alarms and Immobilizers
- Tools and Tool Hire
- Car Care and Valeting
- Cool Air Camping
- Fire Extinquishers
- Propex Heating
- Towing and Tow Bar
- Books and Manuals
- VW Gifts and Merchandise
- Really Miscellaneous Products
- Pertronix Products
- Transporterhaus Products
- BBT Products
- CSP Products
- Vintage Speed Products
Price Match
Now via Live Chat
Free Shipping
When you spend over £75
Free Shipping when you spend over £75
Doesn’t apply to bulky items, click for details
Easy Returns
Just call for a return number
Next Day Delivery
Need it fast?
- > Shop by Vehicle
- > Splitscreen
- > Body, Door and Roof
- > Safari Windows and Popout Windows

Safari Windows and Popout Windows
We have 28 safari windows and popout windows for you.
Complete Kits and Replacement Parts

Part Number: 211898105A
£199.50 inc. VAT
This part fits:
Part Number: 221898321
£37.50 inc. VAT

Part Number: 221847135A
£14.50 inc. VAT

Part Number: 221847131A
£13.50 inc. VAT

Part Number: 221845325B
Offer Ends 29/06/2024
£5.00 inc. VAT

Part Number: 211845123B
£26.50 inc. VAT

Part Number: 211898100
£71.95 inc. VAT

Part Number: 2118475156
£131.15 inc. VAT

Part Number: 211847515
£79.50 inc. VAT

Part Number: 211847516
We have 28 Product(s) that meet your search
We cannot find the specified product information, please click below to view the product page.
View Product
Email me when this product is available
Your Name *
Your Email *
Tell us about your Vehicle and we will tailor the website to you
If you haven’t registered on our website you can save your VW(s) but they will only be available for your current session
WELCOME TO LIMEBUG – AIRCOOLED VW PARTS SPECIALISTS
- Hi, Login or Create Account

T1 1965-72 Beetle Rear Pop-out Safari Window Frame with Glass Kit
In the kit you will receive
Description
Additional information, classic aircooled vw rear safaris parts by limebug.
Safari Frame complete with Glass, With all hardware required for installation, Latches Sliders and Seals
SKU: 080070007
Related Products

1961-72 Beetle / Ghia Complete Gearbox / Trans Mount Kit, w/ Hardware

Adjust-a-Raise Adjustable Gearbox / Trans Raise, Stock to 1″, Raw

Beetle / Ghia Gen-II Shock Towers Titan for Static Beams, 6″ Narrowed Fitment (Also Trekker 2″ Fit), Pair

Beetle / Ghia Bolt on Beam Steering Stop for Ball Joint / Link Pin, Raw

Beetle / Ghia Gen-II Shock Towers Titan for Static Beams Stock – 4″ Narrowed Fitment, Pair

1966-79 Beetle / Ghia 4″ Narrowed Ball Joint Complete IRS Lowering Kit

Upto 72 Rear Gearbox Transmission, Rubber

Beetle / Ghia / Type 3 IRS Rear Lowering Kit

1966-79 Beetle Urethane Shift Coupler / Coupling Gearbox Linkage

Beetle / Ghia Gen-II Shock Towers Jawbreaker for Air Ride Beams, 6″ Narrowed Fitment (Also Trekker 2″ Fit), Pair

1968-79 RHD Beetle Glovebox Air Ride Management Gauge Mount

1972-79 Beetle / Karmann Ghia Gearbox Transmission Mount, Left, Rubber

1948-65 Link Pin Beetle / Ghia Complete Cantilever Swing Axle / IRS Air Ride Kit

1966-79 Beetle / Ghia 4″ Narrowed Ball Joint Deluxe Cantilever Swing Axle / IRS Air Ride Kit

1950-65 Beetle / Ghia 4″ Narrowed Link Pin Complete IRS Lowering Kit

1972-79 Beetle / Karmann Ghia Gearbox Transmission Mount, Right, Rubber
Privacy overview.
Update to the latest version of Safari
If a Safari update is available for your device, you can get it by updating or upgrading macOS, iOS, iPadOS, or visionOS.

Get Safari updates for Mac, iPhone, iPad, or Apple Vision Pro
The most up-to-date version of Safari is included with the latest version of the operating system for your Apple device.
To update Safari on Mac, update macOS .
To update Safari on iPhone or iPad, update iOS or iPadOS .
To update Safari on Apple Vision Pro, update visionOS .
Get Safari updates for Windows
Apple no longer offers Safari updates for Windows or other PC operating systems. Safari 5.1.7 for Windows, released in 2010 and now outdated, was the last version made for Windows.
If a website says your browser is out of date
If a website says that Safari is out of date even though you're already using the latest version of macOS, iOS, iPadOS, or visionOS, there could be an issue with the website. If you’re sure that you want to use the website, contact the website owner or developer for guidance about how to best use their website.

Related topics
Explore Apple Support Community
Find what’s been asked and answered by Apple customers.
- Скидки дня
- Справка и помощь
- Адрес доставки Идет загрузка... Ошибка: повторите попытку ОК
- Продажи
- Список отслеживания Развернуть список отслеживаемых товаров Идет загрузка... Войдите в систему , чтобы просмотреть свои сведения о пользователе
- Краткий обзор
- Недавно просмотренные
- Ставки/предложения
- Список отслеживания
- История покупок
- Купить опять
- Объявления о товарах
- Сохраненные запросы поиска
- Сохраненные продавцы
- Сообщения
- Уведомление
- Развернуть корзину Идет загрузка... Произошла ошибка. Чтобы узнать подробнее, посмотрите корзину.
Oops! Looks like we're having trouble connecting to our server.
Refresh your browser window to try again.
Article Contents
- Materials and Methods
- Acknowledgments
- References Cited
- < Previous
Longhorn Beetle (Coleoptera: Cerambycidae) Assemblage and the Structural Heterogeneity of Habitat at the Brazilian Atlantic Forest
- Article contents
- Figures & tables
- Supplementary Data
Felipe Donateli Gatti, Taís Helena Araujo Rodrigues, Luis Antonio Dias Figueiredo, Marco Antônio Alves Carneiro, Longhorn Beetle (Coleoptera: Cerambycidae) Assemblage and the Structural Heterogeneity of Habitat at the Brazilian Atlantic Forest, Environmental Entomology , Volume 47, Issue 6, December 2018, Pages 1413–1419, https://doi.org/10.1093/ee/nvy158
- Permissions Icon Permissions
The longhorn beetles play important roles in the forest ecosystem processes and their diversity is affected by many environmental disturbances worldwide. This study aimed at understanding how the structural heterogeneity of the habitat can affect the longhorn beetle assemblage in three areas of an Atlantic Forest, which suffered differential impacts in the past and are now in different successional stages. The area in the most advanced successional stage had mainly lower density of trees, but with greater availability of dead wood, especially larger diameter classes. They are important forest components that contribute to the structural diversity of the habitat providing resources for a variety of dependent species. This area has also shown the greatest richness and abundance of longhorn beetles. Our results suggest that these beetles are closely associated with the structural heterogeneity of forests and can be valuable indicators for assessing biodiversity and quality of forest habitat. It also shows that old-growth forest remnants can be the key to the maintenance of the diversity of the longhorn beetles and, consequently, of the ecosystem services they provide.
Email alerts
Citing articles via.
- Advertising and Corporate Services
- Entomology Today
- Recommend to Your Librarian
Affiliations
- Online ISSN 1938-2936
- Print ISSN 0046-225X
- Copyright © 2024 Entomological Society of America
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 03 March 2021
Scale-dependent contribution of host-specificity and environmental factors to wood-boring longhorn beetle community assemblage in SW China
- Fang Luo 1 , 3 ,
- Ling-Zeng Meng 1 , 2 , 5 ,
- S. Tharanga Aluthwattha ORCID: orcid.org/0000-0002-9973-8165 7 ,
- Mei-Ying Lin 4 ,
- Andreas Weigel 6 ,
- Wen-Fu Zhang 1 ,
- Jin-Hua Qi 1 &
- Jin Chen 1
Scientific Reports volume 11 , Article number: 5100 ( 2021 ) Cite this article
775 Accesses
2 Citations
1 Altmetric
Metrics details
- Plant sciences
Longhorn beetles are extremely rich wood-boring insects possessing larvae that feed on the xylem of trees and/or lianas, which have detrimental effects on plants; in turn, the hosting plants may play a fundamental role in shaping the longhorn beetle community assemblage. However, factors determining the community assemblage of wood-boring longhorn beetles, particularly along the multiple spatial scales is still in need of further exploration. In this study, we designed an experiment across several spatial scales (from local to macro scales) from tropical to temperate climate gradients in Yunnan province, southwest China to examine to what extend the attributes of host-specificity is shaping the community assemblage along different spatial scales. This study concludes that (1) the wood-boring longhorn beetles showed attributes of host-specificity to a certain degree at the community level, (2) biotic (host plant specificity) and abiotic (climatic gradients) factors jointly shaped community composition of this species along the multiple spatial scales, (3) biotic interactions have a prominent effect on the community composition of this species at local-scale while macroclimatic gradients impose the major control on it at macro-scale. Thus, this study highlights the significance of host specificity in affecting the wood-boring longhorn beetle community assemblage, particularly at local scales.
Similar content being viewed by others
Attributes of host-specificity better explain the diversified wood-boring longhorn beetles in tropical SW China than plant species diversity
Wood-inhabiting fungal responses to forest naturalness vary among morpho-groups

Niche partitioning among dead wood-dependent beetles
Introduction.
Herbivorous insects occupied nearly a quarter of all terrestrial macroscopic biome on the planet 1 , 2 . The intimate association with terrestrial plants (especially angiosperms) has been considered as the dominating driving force of extraordinary diversification of herbivorous insects 3 , 4 , 5 , 6 . Most herbivorous insects feed on one or few related plant species and showed narrow host-range 7 ; their co-evolution with different host-plant species can potentially generate ecological specialization in plant-feeding insects, and subsequently results in species formation 8 , 9 . If plant diversity shapes insect species that feed on the plants, insect community assemblage should be strongly correlated with the hosting-plant community composition. On the other hand, the distribution of insects is also determined by abiotic factors 10 . To distinguish the contribution of abiotic and biotic factors to the community assemblage has been one of the key issues of community ecological studies for decades 10 , 11 , 12 .
β-Diversity, a method to define the spatial or temporal variation of species composition, has provided insights into the processes that create and maintain community assemblages in the environment 13 , 14 . The β-diversity of plant–phytophagous insect food webs, which relates to the community compositional change related to trophic interactions among food weeb has extended the traditional study of β-diversity at a single trophic level and integrated the spatial turnover of plants and phytophagous insects with changes in the insect–host plant preferences 15 . In general, plants β-diversity patterns are closely related to environmental variation as well as distance per se 16 . However, the β-diversity of herbivorous insects is to a large extent determined by their ability to follow host-plant species in spatiotemporal scales across the different environments. As the species at higher trophic levels are more dependent on species at lower trophic levels, the dependence is determined by the presence of at least one resource species as a prerequisite for the presence of the consumer species 17 , 18 , 19 ; the β-diversity variation of the consumers may result from the species compositional change of the producers, as they create biotic filters for consumers that have close associations with them. Likewise, if the insects are specialist consumers, then the dissimilarity will scale up through the trophic chain 20 , 21 . Specialized consumers are particularly sensitive to compositional changes of resources as their distribution is restricted by host availability 15 . However, this is not necessarily true for generalists, as they have a wider diet-breadth, and their feeding species can be freely distributed and exert no influence on their distribution. The selective pressure exerted by resource hosts is therefore much weaker for generalists than for specialists 15 , 22 .
Although their matching patterns could indicate host specialization of phytophagous insects to their resource plants, it could also indicate parallel responses of both taxa to broad abiotic factors (macro-climatic gradients or shared historical processes). Therefore, it is often difficult to tease apart the potential mechanism of the parallel response of biome to macro-climatic gradients and biogeographic histories to biotic plant–insect interactions 23 . An indirect attempt to distinguish these two forces apart is to examine the patterns of association between plant and insect β-diversity at different spatial scales 24 . Usually, biotic interactions exert their influence at relatively small spatial scales. For example, in a synthesis paper, Pearson and Dawson 11 proposed that biotic interactions were expected to play a role in shaping species distributions over local extents. On the contrary, abiotic factors usually exert their influence at broader spatial scales. Along this prediction, if both patterns result from insect–host specialization, plant and insect β-diversity should be correlated at fine as well as at broad spatial scales; alternatively, if the patterns result from parallel responses to broad abiotic gradients, the β-diversity patterns should only be correlated at broad spatial scales 24 .
Longhorn beetles, belonging to the order Coleoptera (class: Insecta), often play an important role on their hosting plants, damaging hosts’ trunk and even accelerating hosts’ death. By living inside the plant xylem, these beetles undergo development, obtain food and protect themselves against adverse environmental conditions and sustain as natural enemies within their host plants. It is estimated that there are more than 35,000 species of longhorn beetles worldwide, in about 4000 genera 25 . The relationships between longhorn beetles and their host plants are often quite specific, but there is a great range in the breadth of host tree species that might be used by the larvae of different species 26 . Thus, longhorn beetles represent an ideal system for understanding the biotic and abiotic factors affecting insect community assemblage across the different spatial scales.
In this study, we designed an experiment across the multiple spatial scales (from local to macro scales) from tropical to temperate climatic gradients to examine whether the longhorn beetles own the attributes of host-specificity at the community level, and to explore the mechanisms of community assemblage of this species across an increasing spatial scale. We asked the following questions: (1) does the wood-boring longhorn beetle assemblage show any attributes of host-specificity at the community level? (2) To what extend the biotic and abiotic factors are shaping the community composition at different spatial scales? (3) What is the relative importance of biotic and abiotic factors in explaining beetles’ community assemblage along the increasing spatial scales?
Tree and beetle composition
A total of 3290 longhorn beetle individuals were collected and assigned to 296 species as determined by specialists (see Supplementary 1 ), which included 1409 individuals of 212 species from tropical Xishuangbanna, 1630 individuals of 83 species from subtropical Ailaoshan and 251 individuals of 16 species from the temperate Lijiang.
A total of 2183 trees individuals from 214 species were recorded (see Supplementary 2 ). This included 1179 individuals of 135 species from tropical Xishuangbanna, 795 individuals of 60 species from subtropical Ailaoshan and 209 individuals of 18 species from temperate Lijiang.
Species community assemblage change with different spatial scales
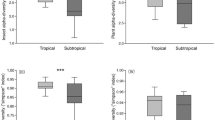
The Wilcoxon paired tests showed that tree communities had a significantly higher β-diversity value (bsor) than those beetles at the scales of β 1 (Z = 2.1668; P < 0.05), β 2 , β 3 and δ 1 (Z = 6.6215, 4.4256 and 9.7828 respectively; P < 0.001) (Fig. 1 ). And at the scale of δ 2 , tree communities and beetle communities showed the same value of bsor = 1.

Insect species (dark grey) and plant species’ (light grey) bsor (overall beta diversity), bnes (nestedness component of bsor), and bsim (replacement component of bsor) was calculated between the survey plots (25 × 20 m 2 ) at different spatial distances: (1) plots within transects (β 1 : 40–100 m scale), (2) plots between two neighboring transects within a region (β 2 : 200–300 m scale), (3) plots between two transects covering the highest elevation gradient within a region (β 3 : 1–3 km scale), (4) plots between two neighboring regions (δ 1 : 250–300 km scale), and (5) plots between two regions covering the highest spatial distance (δ 2 : > 500 km scale). White dots represent medians, thick black bars represent first quartiles and thin black lines represent the range. The shape of each plot shows the frequency distribution of the data. *, ** and ***Significant differences in β-diversity between insects and plants at each spatial scale and region (significance codes: *** P < 0.001, ** P < 0.01, * P < 0.05).
For the replacement component (bsim), we get that tree communities had a significantly higher value than beetles at the scales of β 2 , β 3 and δ 1 (Z = 6.7491, 5.6329 and 3.8215 respectively; P < 0.001), while at the scale of β 1 , tree communities showed no big difference with beetle communities of the replacement index (Z = 1.8599; P > 0.05), At the scale of δ 2 , tree communities and beetles communities showed the same value of bsim = 1.
For the nestedness component (bnes), the tree communities had a significantly lower value than beetles at the scales of β 2 , β 3 and δ 1 (Z = 4.209, 4.6125 and 9.5168 respectively; P < 0.001), while at the scale of β 1 , the nestedness index of tree communities showed no big difference with beetle communities (Z = 0.08612; P > 0.05), and at the scale of δ 2 , tree communities and beetle communities showed the same value of bnes = 0.
Biotic and abiotic drivers of beetle community composition
Variation partitioning of RDA revealed that tree species and tree phylogeny with the joint effect of geographical distance and elevation metrics, separately explained 66% (Fig. 2 A) and 64% (Fig. 2 B) of the variation in beetle community composition, respectively. The pure effect of tree species and tree phylogeny was 12% (Fig. 2 A) and 10% (Fig. 2 B), respectively, while the pure effect of the elevation metrics was 1% (Fig. 2 A) and 1% (Fig. 2 B), separately. Also, the pure effect of geographic distance was 7% (Fig. 2 A) and 12% (Fig. 2 B), respectively.

Variation partitioning results of redundancy analysis testing for the influence of plant community composition, plant phylogeny, environmental variation (elevation metrics, humidity metrics and temperature metrics) and spatial distance on wood-boring longhorn beetle composition in the Yunnan province, SW China. Pla plant species composition, PlaPhy plant phylogeny, Spa spatial distance, Ele elevation metrics, Hum humidity metrics, Tem temperature metrics.
While the tree species and tree phylogeny with the joint effect of geographical distance and humidity metrics, separately explained 65% (Fig. 2 C) and 63% (Fig. 2 D) of the variation in beetle community composition, respectively. The pure effect of tree species and tree phylogeny was 10% (Fig. 2 C) and 8% (Fig. 2 D), respectively. And the pure effect of the humidity metrics was 0% (Fig. 2 C) and 0% (Fig. 2 D), separately. While the pure effect of geographic distance was 5% (Fig. 2 C) and 8% (Fig. 2 D), respectively.
The tree species and tree phylogeny with the joint effect of geographical distance and temperature metrics separately explained 67% (Fig. 2 E) and 62% (Fig. 2 F) of the variation in beetle community composition, respectively. The pure effect of tree species and tree phylogeny was 6% (Fig. 2 E) and 4% (Fig. 2 F). The pure effect of the temperature metrics was 1% (Fig. 2 E) and 0% (Fig. 2 F). While the pure effect of geographic distance was 1% (Fig. 2 E) and 0% (Fig. 2 F). The detailed information about the most important PC axes chosen for explaining beetle community composition is provided in Table S1 .2.
Through linear-mixed effect model, we found that the best model (i.e., delta AIC is equal to 0) retained both plant diversity and environment variables with significant correlation. All residuals of the models showed no significant spatial patterns ( p = 0.34), indicating that our mixed model explicitly incorporated the spatial dependence between plots, transects and regions. The best model showed that fixed effects explained considerable variations of the models with 90.11% (Table 1 ), and the random effect explained 0.04% variation (Table 2 ). For the environment metrics, beetles standardized Simpson diversity is significantly correlated with the standardized minimum temperature of the coldest month (MTCM_stdz) and standardized maximum temperature of the warmest month (MTWM_stdz). For the plant diversity metrics, beetles standardized Simpson diversity is significantly correlated with plant standardized Simpson diversity (PlaSimpson_stdz) and plant standardized phylogenetic diversity (PlaPD_stdz).
Our results reveal that beetles communities composition is highly associated with plant community composition along the multiple spatial scales. The host specificity could be one of the reasons for the close association between plant and insect community composition. This conclusion is verified with the following evidence. Both plant and insect communities exhibit high levels of association (symmetric overall β-diversity distribution, replacement component distribution and nestedness component distribution) across remarkably short spatial scales (i.e., β 1 ) (Fig. 1 ), implying that host plant and insect interaction as the underlying processes, as this is the specific scale range often were the biotic interaction playing role 44 . Besides, the similar pattern of replacement and nestedness components of β-diversity along the increasing spatial extent indicates that these two components played the same vital role in structuring both plant and insect community assemblages, especially at short spatial scales (i.e., β 1 ) (Fig. 1 ). Second, the pure effect of plant species composition as a general control on wood-boring longhorn beetle community composition accounted for 12%, 10% and 6%, separately, of the explained variation (Fig. 2 ), and the plant phylogeny explained 10%, 8% and 2%, respectively (Fig. 2 ). This explanation rate suggests that the relationship between the insects and plants are significantly positively correlated even after removing the effect of geographic distance and environmental metrics, which indirectly indicate that host-plant specificity of Cerambycidae might be one of the driving forces of the presented pattern. Third, from the linear mixed effect model, it is clear that the standardized Simpson diversity of beetles is positively and significantly correlated with standardized plant phylogenetic diversity and plant Simpson diversity, which means that plants community composition and phylogeny are closely associated with insects’ community composition.
In addition to the plant species composition and the phylogeny determined community assemblage of wood-boring longhorn beetles, the environment also played an important role in influencing the wood-boring longhorn beetle community assemblage. From the result of RDA analysis, the effect of the elevation metrics, humidity metrics and temperature metrics to Cerambycidae community assemblage accounted for 11%, 21% and 48%, separately (Fig. 2 ), which indicate the influence of different environmental variations in regulating beetle assemblage with the explaining rate in an order of temperature metrics > humidity metrics > elevation metrics. Also, from linear-mixed effect model, the best model showed that beetles standardized Simpson diversity is positively and significantly correlated with the minimum temperature of the coldest month (MTCM) and the maximum temperature of the warmest month (MTWM) (Table 2 ). All of these suggested that the environmental variation impose constraints on Cerambycidae community assemblage.
Our studies showed that both plant and insect communities exhibit high levels of β-diversity across remarkably short spatial scales (i.e., β 1 ). This is not a pattern expected if compositional co-variation of these groups results from shared bio-geographical histories or parallel responses to climatic gradients 24 , 44 but indicated that host specificity might be the underlying mechanism, particularly at local scales. However, when the spatial scale extends, this highly associated pattern gradually disappeared until it reaches the macro scale (i.e., δ 2 ). Obviously, this highly associated pattern at δ 2 might not be resulted from the plant–insect interaction but following the mechanism as parallel responses of insects and plants to macroclimatic gradients 24 . Additionally, the loose association between insects and plants at the scale of β 2 , β 3 and δ 1 showed clearly that the effect of biotic interactions along the increasing spatial extent gradually disappeared and finally left barely effects at intermediate spatial scales, which impose rarely limitation to coarse-scale beetles’ community assemblage. Thus, we conclude that the dominating mechanisms of insects and tree community assemblage differ at different spatial scales, i.e., at macro-scale, the environmental factors are the major driving forces on longhorn beetle community assemblage, while at the local scale, plant diversity and phylogenetic relationship harbor higher weight on shaping the community assemblage of beetles.
This is not a pattern which only occurs to insects. In nature, the influence of biotic and abiotic factors to biome community assembly are often varying in time and spatial scale. Whittaker proposed a conceptual framework in which abiotic factors (temperature and precipitation) explained the distribution of terrestrial biomes of the world 45 . Furthermore, the idea that climate is the dominant factor shaping species distributions at a broad scale is conceived to explain the correlation of climate and species occurrence patterns observed at a comparable spatial resolution 10 . With difference to the broad spatial scale, Soberón and Nakamura 12 claimed that the pattern of fine spatial resolution is created by biotic interactions. This idea is verified by Pearson and Dawson 11 who stated that biotic interactions are expected to play a role in shaping species distributions only over local extents. All these perspectives imply that the key point of the comparative influence of biotic and abiotic process for species community assemblage rely on the scale.
With this, our study has demonstrated that biotic interactions possess prominent effect at local scale but just create statistic noise within the periphery of wood-boring longhorn beetles community assemblage at macro-scale 46 . Meanwhile, macroclimatic gradients seem to impose the most control on species distribution when reaching coarse scale. This phenomenon was proposed as early by Eltonian niche concept 47 and Grinnellian niche concept 48 . From Eltonian’s 47 point of view, ecological interactions and resource dynamics determine species distributions at fine scales, whereas abiotic factors (climatic gradients) determine species distributions at broader scales 48 . This phenomenon revealed that “niche” relation exhibit a wide spectrum in the natural world, such that macroclimatic environment regulates the possible suitable areas which cater for the intrinsic attributes of a species, whereas biotic variations determine the subset of these areas which remains suitable after considering the resource dynamic limitations and species interactions.
In conclusion, after a series of analyses, this study demonstrates that the wood-boring longhorn beetles own the attributes of host-specificity at community level, and this is the prerequisite of the existence of this species at any spatial scales. At the local scale, longhorn beetles exhibit its strongest biotic niche relations in affecting or being affected by plant species. However, with the increase of spatial resolution, their relationship is expected to be averaged out at broader scales and macroclimatic heterogeneity would dominate the community assemblage processes, and hence the biotic interaction might remain embedded in the macro-scale environmental surroundings and their influence can be deemed as a subset of environmental signals.
Study sites from tropical to temperate regions
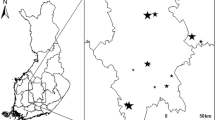
The study was carried out in SW China, the map is generated in R 3.4.5, and the Sampling topographic map is generated with Google earth ( http://earth.google.com ) (Fig. 3 ). This region is well known as one among the global biodiversity hotspots 27 . Owing to the effects of a tropical monsoonal climate and varied mountain hilly topography, with extending Himalayan Mountain range in the southeast, this area is covered with various types of highly complex vegetation from tropical monsoonal rainforest to temperate coniferous forest. Our sampling sites were located at tropical Xishuangbanna, subtropical Ailaoshan and the temperate Lijiang (Fig. 3 ). For each site, we located three transects for both plant and insect survey, allowing the interval of each transect about 200 m differing in altitude with about 0.5–1.5 km in distance.

Geographical location of the three sampling sites, nine sampling transects and 45 sampling plots in the east Himalayan Mountains, Yunnan province, Southwest China, the basic map is generated in R 3.4.5, and ‘.shp’ file is from the open resources of National Catalogue of Service For Geographic Information ( https://www.webmap.cn/main.do?method=index ) under the regulations of Surveying and Mapping Law of the People's Republic of China (2017), and the sampling topographic map is generated with Google earth (Google Earth Pro 7.3.3.7786 (64-bit)) with the permission of GOOGLE TERMS OF SERVICE ( https://policies.google.com/terms?hl=en ).
The tropical site is located in Xishuangbanna (21.61° N, 101.58° S). The mean annual temperature and rainfall at altitude 600 m are about 22 °C and 1500 mm, respectively. The rainy season ranges from May to October and dry season ranges from November to April. Approximately 80% of annual precipitation occurs in the rainy season. Three transects at different elevations (600 m, 800 m and 1000 m) were selected with about 0.5 km distance between two adjacent transects.
The subtropical site was located in Ailao mountains (24.53° N, 101.03° S), which is about 330-km away from Xishuangbanna site (Fig. 3 ). The mean annual temperature and rainfall were 11 °C and 1900 mm, respectively, with a dry season from December to April. This area encompasses evergreen broad-leaved forests primarily dominated by Lithocarpus and Castanopsis at ca. 2200–2600 m a.s.l. with sparse or a dense understory of bamboo and Rhododendron dwarf forests towards the higher elevations. We established three transects at different elevations, i.e., 2200 m, 2400 m and 2600 m, respectively. The distance between two adjacent transects was about 1.5 km.
The temperate site was located at Lijiang (27.14° N, 100.23° S), Yunnan province. The climate of this area has an average annual temperature of 5.5 °C (minimum–maximum), with average annual rainfall around 1600 mm. This area encompasses temperate coniferous forests primarily dominated by Berberidaceae, Caprifoliaceae and Rosaceae as the understory. Both Pinaceae and Fagaceae plants dominate the canopy of the forest. Similar to the transects in tropical and subtropical sites, three transects at three different altitudes, i.e., 3200 m, 3400 m and 3600 m respectively were selected for insect sampling and vegetation inventory. The distance between two adjacent transects was about 1.3 km.
Insect sampling and tree species survey
A spatially nested sampling approach was established along the three different sites. For each transect in the different site, we established five forest plots with a size of 25 × 20 m 2 for the installation of beetle collection devices. The interval distance for two plots was > 40 m.
Beetle sampling was conducted using flight intercept traps (FITs) in the canopy and understory of each forest plot in all the sites to include more species, in case of a community species compositional difference between the canopy and understory exist 28 , 29 . FITs as an effective method to capture Cerambycidae 30 , were constructed with two pieces of hard plastic plates (50 × 35 cm, height × width) which were fixed crosswise and installed upon a yellow plastic bowl (35 × 30 cm, diameter × height). A piece of round, transparent, soft plastic plate with a diameter of 45 cm roofed the top of each FIT to prevent the entry of rainwater during the rainy season. Within each plot, one trap was installed on canopy tree branches at a height of 10–30 m above the ground, and the second one was placed at the understory at a height of 1 m. The collecting basins of the FITs were filled with a liquid mixture of 75% ethanol and anti-freeze (ethylene glycol) at 1:2 v/v. Ethanol is used as lure to attract cerambycidae 31 , and also to prevent decaying the collected insects, while the anti-freeze is used to prevent the liquid freeze when ambient temperature drop below zero. Ten FITs were used in each transect, thereby in total 90 FITs were installed in all the three sampling sites.
Fieldwork at Xishuangbanna was started from April 2018 and ended in April 2019, while at Ailaoshan and Lijiang, it was started from May 2018 and end in May 2019. Traps were emptied once in every 10 days interval. The collected specimen preserved in alcohol and anti-freeze mixture were filtered and preserved in 70% ethanol liquid. Considering the difficulty of identifying beetles into species, they were identified as morphospecies. Voucher specimens of the collected beetles have been deposited temporarily at the laboratory in the Honghe University and the specimens will be finally transferred to the National Zoological Museum of China, Institute of Zoology, Chinese Academy of Sciences, Beijing.
Collection of vegetation data was conducted during April and May 2019 at the same plot corresponding to insect sampling location. We censused woody plants in 0.25-ha plots using identical field methods at each elevation transect (20 m × 25 m × 5 plots). In each plot, we measured the abundance of each tree species (or morpho-species) ≥ 5.0 cm diameter at breast height (1.2 m). All sampling methods used in the present study comply with the instruction of the Center for Tropical Forest Science ( http://www.ctfs.si.edu/ ) to assemble long-term, large-scale forest data from the tropics and the Chinese Forest Biodiversity Monitoring Network ( http://www.cfbiodiv.org/ ). Voucher specimens were collected whenever necessary in the field for later identification with the help of experienced botanists. While establishing plots on slopes, we positioned the plot centerline perpendicular to slopes to minimize the elevation gradients within plots.
Climatic data collection
We recorded air temperature and humidity data at a half-hour frequency using a thermo-logger (DS1923Hygrochron iButton, Maxim, CA, USA) from April 2018 to May 2019, and the duration was the same as the period of the insect collection. The environment data logger device was fixed along with one of the five canopy FITs in each transect. In total, we used seven variables including annual mean temperature (AMT), annual mean humidity (AMH), annual temperature range (ATR), annual humidity range (AHR), maximum temperature of the warmest month (MTWM), minimum temperature of the coldest month (MTCM) and average elevation (ELE) of each transect as the main environmental filter factors. These data were assembled as a secondary environment matrix and detailed data information is listed in Table S1 .1.
Data analyses
Insect and tree diversity estimation.
For beetle diversity, both canopy and understory FITs within each plot were combined as the smallest sampling unit for diversity estimation and the tree diversity was recorded for each plot. We estimated α-diversity with simpson index as the number of species recorded in each sampling unit (Supplementary 1 , 2 ). The selection of a Simpson diversity index is not biased by richness variations allowing affirmation a priori that richness gradients do not bias the present results 32 .
The β-diversity was calculated as Sorenson index (bsor) uses the function ‘beta.pair’ in package ‘betapart’ 33 in R. This method was presented for pairwise and multiple-site comparisons 33 , 34 . In the pairwise situation, an index of beta diversity due to nestedness (bnes), which is deemed to represent richness differences among nested communities, is calculated by subtracting bsim (replacement) from bsor (overall beta diversity). Moreover, index bsim measure used in Baselga’s 33 approach is deemed to reflect compositional differences attributable to replacement, the details about these two indexes are described in Carvalho et al. 35 .
In addition, geographic distance matrices were calculated using the function ‘earth.dist’ in the R package ‘fossil’ at the plot, transect and site levels 36 . The species accumulation curves have approached an asymptote in either of the two sample taxa (Fig. S1 ), also because the consistent sampling units throughout the three sites, this curve may not be that important.
For the plant phylogenetic α-diversity, the family and genus names of all the enumerated species (215 species in total) in the APG III system were obtained with the R package ‘plantlist’ 37 . Then, their phylogenetic relationships were examined using the online phylomatic tool 38 . ( www.phylodiversity.net/phylomatic/ ) based on the Angiosperm consensus tree from Davies et al. 39 . The phylogenetic α-diversity was calculated with ‘pd’ in ‘picante’ in R. ‘pd’ is the sum of the total phylogenetic branch length for the sample 40 .
Spatial scale of species community assemblage
To quantify species β-diversity in relation to spatial distance, by refer to Kemp et al. 24 , the grouped plot-level β-diversity matrix was calculated and then partitioned into various independent spatial components that reflect various β-diversity levels. We calculated bsor (overall beta diversity), bnes (nestedness component of bsor), and bsim (replacement component of bsor) of insect and plant separately along a series of spatial scales: (1) plots within transects (β 1 : 40–160 m scale), (2) plots between two neighboring transects within a site (β 2 : 0.5–1.5 km scale), (3) plots between two transects covering the highest elevation gradient within a site (β 3 : 1–3 km scale), (4) plots between two neighboring sites (δ 1 : 250–300 km scale) and (5) plots between two regions covering the highest spatial distance (δ 2 : > 500 km scale). Here δ-diversity refers to geographic diversity differentiation, i.e., dimensionless comparative number of species applied to changes over large scales, which is the functional equivalent of β-diversity at the higher organizational level of the landscape 41 . Wilcoxon paired tests were used to assess the similarity of β-diversity for trees and beetles at each respective spatial scales (i.e., β 1 , β 2 , β 3 , δ 1 and δ 2 ). P values were adjusted accordingly.
Correlation of biotic and abiotic factors to insect community composition
We used an ordination (redundancy analysis; RDA) approach to analyze tree species composition and tree’s phylogeny combined with environmental variation (divided into three groups: elevation metrics, temperature metrics and humidity metrics) and spatial distance in explaining the beetle composition. The PC axes selected for plant species was conducted with ‘prcomp’ function in ‘stats’ package, while the PC axes selected for plant Phylogeny was conducted with ‘phyl.pca’ function in ‘phytools’ package (Table S1 .1). Forward selection was conducted to assess the influence of different groups variables on beetle composition (‘ordistep’ function in the ‘vegan’ package). Variation partitioning analysis (‘varpart’ function in ‘packfor’ package) after redundancy analysis was performed to assess the percentage contribution (both unique and shared) of each group of predictor variables to explain the variation in abundance of longhorn beetle species composition. The environmental variables were log-normalized, and the spatial distance was converted into the Cartesian coordination for the above calculation. Significance of testable fractions ( P ≤ 0.05) was based on 999 permutations.
Finally, we introduced linear mixed-effect model to analyze the effect of plant α-diversity and phylogenetic α-diversity and environmental variability on beetle α-diversity, respectively. In total we considered three groups of datasets: dataset 1) beetles standardized Simpson diversity (BeeSimpson_stdz); dataset 2) plant standardized diversity index, which including standardized Simpson diversity (PlaSimpson_stdz), and standardized phylogenetic α-diversity (PlaPD_stdz); dataset 3) standardized environmental variability, which including standardized MTWM (MTWM_stdz) and standardized MTCM (MTCM_stdz), these two variables were retained after removing the other environmental variables with correlation index > 0.5. Standardized Simpson diversity index of beetle diversity was treated as the response variable and transects nested inside site names were treated as a random effect, and the remaining variables including dataset 2) and dataset 3) were treated as the fixed effects. Moran's I correlogram was built to evaluate the degree of spatial autocorrelation of the variables in relation to geographic distances and we found no significant positive spatial autocorrelation for these variables. For each dataset, we first fitted one global model (BeeSimpson_stdz ~ PlaPD_stdz + PlaSimpson_stdz + MTWM_stdz + MTCM_stdz + (1|SiteName/Transect)). The ‘dredge’ function in the ‘MuMIn’ R package was used to fit all the possible combinations of models nested in the global models. Model selection was performed based on Akaike Information Criterion values (AICc) corrected for small sample size 42 . After a series of global model replication,we got a number of submodels but only retained the top-ranking one. All candidate models with delta < 2 are presented. All the analyses were performed using R 3.4.5 43 .
Strong, D. R., Lawton, J. H. & Southwood, S. R. Insects on Plants, Community Patterns and Mechanisms (Blackwell Scientific Publicatons, Hoboken, 1984).
Google Scholar
Daly, H. V., Doyen, J. T. & Purcell, A. H. Introduction to Insect Biology and Diversity (Oxford University Press, Oxford, 1998).
Strong, D. R., Lawton, J. H. & Southwood, T. R. E. Insects on Plants: Community Patterns and Mechanisms (Harvard University Press, Cambridge, 1984).
Mitter, C., Farrell, B. D. & Wiegmann, B. The phylogenetic study of adaptive zones: Has phytophagy promoted insect diversification?. Am. Nat 132 , 107–128 (1988).
Article Google Scholar
Farrell, B. D. “Inordinate fondness” explained: Why are there so many beetles?. Science 281 , 555–559 (1998).
Article CAS PubMed Google Scholar
Marvaldi, A. E., Sequeira, A. S., O’Brien, C. W. & Farrell, B. D. Molecular and morphological phylogenetics of weevils (Coleoptera, Curculionoidea): Do niche shifts accompany diversification?. Syst. Biol 51 , 761–785 (2002).
Article PubMed Google Scholar
Futuyma, D. J. Evolution of Host Specificity in Herbivorous Insects: Genetic, Ecological, and Phylogenetic Aspects (Wiley, New York, 1991).
Via, S., Bouck, A. C. & Skillman, S. Reproductive isolation between divergent races of pea aphids on two hosts. II. Selection against migrants and hybrids in the parental environments. Evolution 54 , 1626–1637 (2000).
Nosil, P. Transition rates between specialization and generalization in phytophagous insects. Evolution 56 , 1701–1706 (2002).
Woodward, F. I. Climate and Plant Distribution (Cambridge University Press, Cambridge, 1987).
Pearson, R. G. & Dawson, T. P. Predicting the impacts of climate change on the distribution of species: Are bioclimate envelope models useful?. Global. Ecol. Biogeogr 12 , 361–371 (2003).
Soberón, J. & Nakamura, M. Niches and distributional areas: Concepts, methods, and assumptions. Proc. Natl. Acad. Sci 106 , 19644–19650 (2009).
Article ADS PubMed Google Scholar
Whittaker, R. H. Evolution and measurement of species diversity. Taxon 21 , 213–251 (1972).
Vellend, M. Conceptual synthesis in community ecology. Q Rev. Biol 85 , 183–206 (2010).
Novotny, V. Beta diversity of plant-insect food webs in tropical forests: A conceptual framework. Insect. Conserv. Diver 2 , 5–9 (2009).
Legendre, P., Borcard, D. & Peres-Neto, P. R. Analyzing beta diversity: Partitioning the spatial variation of community composition data. Ecol. Monogr 75 , 435–450 (2005).
Price, P. W. Resource-driven terrestrial interaction webs. Ecol. Res 17 , 241–247 (2002).
Tscharntke, T. & Hawkins, B. A. Multi-Trophic Level Interactions (Cambridge University Press, Cambridge, 2002).
Book Google Scholar
Pereira, M. L., Matos, M. A., Lewinsohn, T. M. & Almeida, N. M. Trophic level and host specialisation affect beta-diversity in plant–herbivore–parasitoid assemblages. Insect. Conserv. Diver 12 , 404–413 (2019).
Novotny, V. & Weiblen, G. D. From communities to continents: Beta diversity of herbivorous insects. Ann. Zool. Fenn 42 , 463–475 (2005).
Lewinsohn, T. M. & Roslin, T. Four ways towards tropical herbivore megadiversity. Ecol. Lett 11 , 398–416 (2008).
Ødegaard, F. Host specificity, alpha- and beta-diversity of phytophagous beetles in two tropical forests in Panama. Biodivers. Conserv 15 , 83–105 (2006).
Hawkins, B. A. & Porter, E. E. Does herbivore diversity depend on plant diversity? The case of California butterflies. Am. Nat. 161 , 40–49 (2003).
Kemp, J. E., Linder, H. P. & Ellis, A. G. Beta diversity of herbivorous insects is coupled to high species and phylogenetic turnover of plant communities across short spatial scales in the Cape Floristic Region. J. Biogeogr. 44 , 1813–1823 (2017).
Lawrence, J. F. Synopsis and Classification of Living Organisms, ***Vol 2 (McGraw-Hill, New York, 1982).
Hanks, L. M. Influence of the larval host plant on reproductive strategies of cerambycid beetles. Annu. Rev. Entomol. 44 , 483–505 (1999).
Myers, N., Mittermeier, R. A., Mittermeier, C. G., Fonseca, G. A. B. & Kent, J. Biodiversity hotspots for conservation priorities. Nature 403 , 853–857 (2000).
Article ADS CAS PubMed Google Scholar
Corff, J. & Marquis, R. Difference between understory and canopy in herbivore community composition and leaf quantity for two oak species in Missouri. Ecol. Entomol. 24 , 46–58 (2001).
Ulyshen, M. D. & Hanula, J. L. A comparison of the beetle (Coleoptera) fauna captured at two heights above the ground in a North American temperate deciduous forest. Am. Midl. Nat. 158 , 260–278 (2007).
Groot, P. & Nott, R. Evaluation of traps of six different designs to capture pine sawyer beetles (Coleoptera: Cerambycidae). Agric. Forest. Entomol 3 , 107–111 (2002).
Miller, D. et al. Ipsenol, ipsdienol, ethanol, and pinene: Trap lure blend for Cerambycidae and Buprestidae (Coleoptera) in Pine Forests of Eastern North America. J. Econ. Entomol. 20 , 20 (2015).
Simpson, E. H. Measurement of diversity. Nature 163 , 688 (1949).
Article ADS MATH Google Scholar
Baselga, A. Partitioning the turnover and nestedness components of beta diversity. Global. Ecol. Biogeogr 19 , 134–143 (2010).
Baselga, A. The relationship between species replacement, dissimilarity derived from nestedness, and nestedness. Glob. Ecol. Biogeogr 21 , 1223–1232 (2012).
Carvalho, J. C. et al. Determining the relative roles of species replacement and species richness differences in generating beta-diversity patterns. Glob. Ecol. Biogeogr. 21 , 760–771 (2012).
Vavrek, M. J. fossil: Palaeoecological and palaeogeographical analysis tools. Palaeontol. Electron 14 , 1 (2011).
Zhang, J.-L. Plantlist: Looking Up the Status of Plant Scientific Names based on The Plant List Database (Version 0.3.7) (2018).
Webb, C. O. & Donoghue, M. J. Phylomatic: Tree assembly for applied phylogenetics. Mol. Ecol. Notes 5 , 181–183 (2005).
Davis, T. J. et al. Darwin’s abominable mystery: Insights from a supertree of the angiosperms. Proc. Natl. Acad. Sci 101 , 1904–1909 (2004).
Article ADS CAS Google Scholar
Kembel, S. W. et al. Picante: R tools for integrating phylogenies and ecology. Bioinformatics 26 , 1463–1464 (2010).
Whittaker, R. H. Vegetation of the Siskiyou Mountains, Oregon and California. Ecol. Monogr 30 , 279–338 (1960).
Burnham, K. P. & Anderson, D. R. Model Selection and Multimodel Inference (Springer, Berlin, 2002).
MATH Google Scholar
R Core Team R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna . https://www.R-project.org (2018).
Chesson, P. Mechanisms of maintenance of species diversity. Annu. Rev. Ecol. Evol. S 31 , 343–366 (2000).
Whittaker, R. J. Communities and Ecosystems (MacMillan, New York, 1975).
Araújo, M. B. & Luoto, M. The importance of biotic interactions for modelling species distributions under climate change. Glob. Ecol. Biogeogr 16 , 743–753 (2007).
Elton, C. S. Animal Ecology (Sedgwick & Jackson Ltd, London, 1927).
Grinnell, J. The niche-relationships of the California Thrasher. Auk 34 , 427–433 (1917).
Download references
Acknowledgements
We thank Yun-Meng Wang, Hua-Yue Ma, Kun-Fu Chen, and Shan Sun at the Honghe University for help in sorting of morph species samples in the laboratory. Jin-Long Zhang at the Kadoorie Farm and Botanic Garden (KFBG) of China, Hongkong provided useful suggestions for statistical methods. Mahandran Valliyappan at Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences provided English language editing work. This study was supported by the West Light Foundation of the Chinese Academy of Sciences and the CAS 135 program (No. 2017XTBG‐T01), funds from the National Natural Science Foundation of China (Grant no. NSFC-31200322; NSFC-31760171; 31870359 and U1402264) and Honghe University (Grant no. XJ16B05).
Author information
Authors and affiliations.
CAS Key Laboratory of Tropical Forest Ecology, Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences, Mengla, 666303, Yunnan, China
Fang Luo, Ling-Zeng Meng, Wen-Fu Zhang, Jin-Hua Qi & Jin Chen
College of Life Science and Technology, Honghe University, Mengzi, 661199, Yunnan, China
Ling-Zeng Meng
University of Chinese Academy of Sciences, Beijing, 100049, China
Institute of Zoology, Chinese Academy of Sciences, Beijing, 100101, China
Mei-Ying Lin
University of Hohenheim, Institute of Agricultural Sciences in the Tropics (Hans-Ruthenberg-Institute) (490f), 70593, Stuttgart, Germany
Natural History Museum of Erfurt, Große Arche 14, 99084, Erfurt, Germany
Andreas Weigel
Guangxi Key Laboratory of Forest Ecology and Conservation, College of Forestry, Guangxi University, Daxuedonglu 100, Nanning, 530004, Guangxi, China
S. Tharanga Aluthwattha
You can also search for this author in PubMed Google Scholar
Contributions
J.C. and F.L. conceived the ideas. F.L. and L.Z.M. collected the data. A.W. and M.Y. L. classified the Cerambycidae. J.H.Q. and W.F.Z. classified the plants. F.L. and S.T.A. analyzed the data with support from L.Z.M. and C.J. and F. L. wrote the manuscript. All authors commented and gave final approval for publication.
Corresponding author
Correspondence to Jin Chen .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary information 1., supplementary information 2., supplementary information 3., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Luo, F., Meng, LZ., Aluthwattha, S.T. et al. Scale-dependent contribution of host-specificity and environmental factors to wood-boring longhorn beetle community assemblage in SW China. Sci Rep 11 , 5100 (2021). https://doi.org/10.1038/s41598-021-84511-3
Download citation
Received : 15 October 2020
Accepted : 08 February 2021
Published : 03 March 2021
DOI : https://doi.org/10.1038/s41598-021-84511-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Spatial distribution patterns of longhorn beetle assemblages (coleoptera: cerambycidae) in mongolian oak forests in changbai mountains, northeast, china.
- Yinghua Jin
Journal of Insect Conservation (2024)
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Anthropocene newsletter — what matters in anthropocene research, free to your inbox weekly.
The specific assemblage structure of longhorn beetles (Coleoptera, Cerambycidae) in floodplain forests of the western part of Saratov oblast
- Published: 28 March 2017
- Volume 43 , pages 1416–1421, ( 2016 )
Cite this article

- V. P. Gorshkova 1 &
- A. N. Volodchenko 2
42 Accesses
Explore all metrics
The biodiversity structure and habitat requirements of longhorn beetles (Cerambycidae) in floodplain forests of the western part of Saratov oblast were studied from 2011 to 2014. A total of 51 species of longhorn beetles has been identified. The largest subfamilies are Cerambycinae (19 species), Lepturinae (17 species), and Lamiinae (13 species). The specific communities include 34, 14, 11, 9, 7, 7, 6, and 3 species for oak, aspen, elm, willow, linden, maple, alder, and ash tree, respectively. The largest number of longhorn beetle species was found in oak forests.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others
A comparison of diversity and species composition of ground beetles (coleoptera: carabidae) between conifer plantations and regenerating forests in korea.

Communities of phyllophagous insects in young birch greeneries of northern cities
Dung beetle communities of altitudinal Atlantic forest remnants: diversity and composition
Danilevskii, M.L. and Miroshnikov, A.I., Zhuki-usachi Kavkaza (Coleoptera, Cerambycidae) (Capricorn Beetles (Coleoptera, Cerambycidae) of the Caucasus), Krasnodar: Izd. Kubansk. S.-Kh. Inst., 1985.
Google Scholar
Gamayunova, S.G. and Novak, L.V., On the problem of colonization of dead wood of English oak with xylophages, Lisivnitstvo Agrolisomelioratsiya (Kharkiv) , 2009, vol. 115, pp. 261–267.
Kalyuzhnaya, N.S., Komarov, E.V., and Cherezova, L.B., Zhestkokrylye nasekomye Nizhnego Povolzh’ya (Coleoptera of the Lower Volga Region), Volgograd: Region. Tsentr po Izucheniyu i Sokhraneniyu Bioraznoobraziya, 2000.
Kovalenko, Ya.N., Study of xylobiontic beetles (Coleoptera) of the southern part of the Central Russian forest stepper associated with the species of the genus Populus, Nauch. Vedom. Belgorodsk. Gos. Univ., Estestv. Nauki , 2010, vol. 21, no. 13, pp. 62–68.
Lindeman, G.V., Colonization of deciduous trees by stem pests in oak forests in the forest steppe zone as a result of their weakening and dying off (a case study of Tellerman forest), in Zashchita lesa ot vrednykh nasekomykh (Forest Protection from Pests), Moscow: Nauka, 1964, pp. 58–116.
Lindeman, G.V., Zaselenie duba stvolovymi vreditelyami v svyazi s oslableniem i otmiraniem v dubravakh lesostepi (a case study of Tellerman forest), in Vliyanie zhivotnykh na produktivnost’ lesnykh biogeotsenozov (The Effect of Animals on the Productivity of Forest Biogeocenoses), Moscow: Nauka, 1966, pp. 75–96.
Mamaev, B.M., Biology of wood-destructing insects, in Itogi nauki i tekhniki. Entomologiya (Advances in Science and Technology, Ser. Entomology), Moscow: VINITI, 1977, vol. 3.
Pesenko, Yu.A., Printsipy i metody kolichestvennogo analiza v faunisticheskikh issledovaniyakh (Principles and Methods of Quantitative Analysis in Faunal Studies), Moscow: Nauka, 1982.
Plavil’shchikov, N.N., Fauna SSSR. Nasekomye zhestkokrylye (Fauna of the USSR. Coleoptera), vol. 21: Zhuki-drovoseki (Capricorn Beetles), Moscow: Izd. AN SSSR, 1936, part 1.
Plavil’shchikov, N.N., Fauna SSSR. Nasekomye zhestkokrylye (Fauna of the USSR. Coleoptera), vol. 22: Zhuki-drovoseki (Capricorn Beetles), Moscow: Izd. AN SSSR, 1940, part 2.
Plavil’shchikov, N.N., Fauna SSSR. Nasekomye zhestkokrylye (Fauna of the USSR. Coleoptera), vol. 23: Zhuki-drovoseki (Capricorn Beetles), Moscow: Izd. AN SSSR, 1958, part. 3.
Shapovalov, A.M., Longhorn beetles (Coleoptera, Cerambycidae) of the Orenburg oblast: fauna, distribution, and bionomics, Tr. Orenb. Otd. Rus. Entomol. Obshch. , 2012, no. 3, pp. 1–221.
Sukneva, V.P., Analysis of the zoogeographic structure of the fauna of longhorn beetles (Coleoptera, Cerambicidae) of the Saratov oblast, in Aktual’nye problemy nauki i obrazovaniya: sb. nauch. st. (Actual Problems of Science and Education: Collected Scientific Papers), Balashov: Nikolaev, 2012, pp. 107–108.
Sukneva, V.P., Biodiversity of longhorn beetles of the Cis- Khoper area of the Saratov oblast, in Issledovaniya v oblasti estestvennykh nauk i obrazovaniya: sb. nauch.-issled. rabot studentov (Research in Science and Education: Collected Scientific Papers of Students), Samara: Porto-print , 2013, vol. 3, pp. 95–99.
Volodchenko, A.N., On the study of the fauna of longhorn beetles (Coleoptera, Cerambicidae) of the Middle Khoper region, in Gorod i ekologiya: materialy mezhregion. nauch.- prakt. konf. (City and Ecology: Proc. Interregional Sci.- Pract. Conf.), Voronezh: Krivichi, 2008, pp. 158–163.
Volodchenko, A.N., Succession complexes of xylobiont coleopterans of the deciduous forests of the Middle Cis- Khoper region, Izv. S.-Peterb. Lesotekhn. Akad. , 2009, no. 187, pp. 79–86.
Volodchenko, A.N., On the study of xylophagous coleopterans of plantations of the Saratov oblast, in VII Chteniya pamyati O.A. Kataeva. Vrediteli i bolezni drevesnykh rastenii Rossii: materialy mezhdunar. konf. (VII Lectures in Memory of O.A. Kataev. Pests and Diseases of Woody Plants of Russia: Proc. Int. Conf.), St. Petersburg: Izd. SPbGLTU , 2013.
Vorontsov, A.I., Gur’yanova, T.M., and Mozolevskaya, E.G., A review of harmful forest insects of the Khoper Reserve, Tr. Khoper. Zapov. , 1961, no. 4, pp. 47–74.
Zolotukhin, A.I. and Ovcharenko, A.A., Poimennye lesa Prikhoper’ya: sostoyanie, ekologo-tsenoticheskaya struktura, bioraznoobrazie (Floodplain Forests of the Cis-Khoper Region: Status, Ecological and Cenotic Structure, and Biodiversity), Balashov: Nikolaev, 2007.
Zolotukhin, A.I., Ovcharenko, A.A., Zanina, M.A., and Shapovalova, A.A., Ecological and cenotic characteristics and dynamics of floodplain oak forests of the Cis-Khoper region, Povolzh. Ekol. Zh. , 2011, no. 3, pp. 314–322.
Download references
Author information
Authors and affiliations.
Saratov State Technical University, ul. Politekhnicheskaya 77, Saratov, 410054, Russia
V. P. Gorshkova
Balashov Institute, Chernyshevskii Saratov State University, ul. K. Marksa 29, Balashev, Saratov oblast, 412300, Russia
A. N. Volodchenko
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to V. P. Gorshkova .
Additional information
Original Russian Text © V.P. Gorshkova, A.N. Volodchenko, 2015, published in Povolzhskii Ekologicheskii Zhurnal, 2015, No. 4, pp. 381–389.
Rights and permissions
Reprints and permissions
About this article
Gorshkova, V.P., Volodchenko, A.N. The specific assemblage structure of longhorn beetles (Coleoptera, Cerambycidae) in floodplain forests of the western part of Saratov oblast. Biol Bull Russ Acad Sci 43 , 1416–1421 (2016). https://doi.org/10.1134/S1062359016100058
Download citation
Received : 24 February 2015
Published : 28 March 2017
Issue Date : December 2016
DOI : https://doi.org/10.1134/S1062359016100058
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Cerambycidae
- floodplain forests
- habitat preference
- steppe zone
- Saratov oblast
- Find a journal
- Publish with us
- Track your research

IMAGES
VIDEO
COMMENTS
VW Bug Safari windows by VW LooseNuts: Our VW Safari window kits are Hand Crafted In The USA and we are dedicated to quality, providing custom VW window accessories including Front Safaris, Side PopOuts, Rear Type 1 Safaris, Oval Window Type 1 Rear Safaris, Convertible Front Safaris, and Caddy Rear Safaris. Parts Store ...
Posted: Thu Oct 29, 2020 7:49 am Post subject: Re: Safari Window (front) Beetle. I paid $650 for my 67 with a chrome finish. I think they're around $500 for raw steel. I've had mine installed for around 12,000 miles, and honestly I've only driven with it closed for about 700 of those miles.
This Bug Safari window is constructed as a dual-channel, mild-steel frame with a chrome finish that accepts 3/16" safety glass and an outer lip seal. This chrome options arrives pre-assembled. Application: Bug (Type 1): 58-64. Off-Road: 58-64. $1,238.18.
1958-1964 us spec vw bug front safari window ** for australian spec cars 58-67** (pre order) from $500.00 safari frame, with all the hardware, latches, sliders and seals. ***you use your stock glass if your glass is not og glass the inner seal provided may not work. there is another seal that can be used if aftermarket glass is to tight with ...
Samba Member. Joined: May 17, 2011. Posts: 90. Location: Chattanooga, Tennessee. Posted: Thu Nov 03, 2011 6:23 pm Post subject: Bug Safari Window Kit... Experiences? Info? Ive been considering that retrofit of a non-stock but bolt in/out... the Safari Window (windshield) kit for 1958-64 Bugs offered by Wolfgang International (and others?).
Sell now. VW Beetle Bug Front Safari Window For 1965-1967 Slightly / Triple Chrome Plating. kaefer-nostalgie. (254) 98.1% positive. Seller's other itemsSeller's other items. Contact seller. US $849.00. $35.37 for 24 months with PayPal Credit *.
After many years leading the Safari Window market for Beetle and Bus we are very glad to present the first available production of the very rare and sought after Slightly Curved front Safari Windows.
1950-52 Split Window Beetle and 1952/57 Early and Late Oval Beetle use the same Safari Windows, remember these Beetles use the same big Sekurit 5mm thickness glass in all these years, so you will find the Ad as Split Oval Safari Windows on our store. 1958-64 Beetles use the well known flat glass Safari, inches height aprox. 15 inches (the ...
SAFARI FRONT WINDOW POP OUT KIT, STANDARD BUG SEDAN 1968-77, SUPER BEETLE SEDAN 1971-72, CHROME FRAME, SLIDERS, LATCHES, SEALS & HARDWARE *MADE IN USA*. Set. Item #: 361-200. Price: $799.95. In Stock. Qty: Add to Cart. Items 1-1 of 1. Shop Safari Window Kits & Parts for a 1968 VW Bug Sedan at West Coast Metric - home for all of your VW Bug ...
New Safari Window kit. Polished stainless steel *Kit does not include wiper shafts or one eyed duck Made in USA. Fits: 1955-1967 Type 2 Bus (all models) ... BEETLE SPLIT WINDOW BUS SPLIT WINDOW BUS BAYWINDOW BAYWINDOW TYPE 3 TYPE 3 GHIA GHIA TYPE 3 TYPE 3 TYPE 34 ...
Find many great new & used options and get the best deals for VW Beetle Bug Rear Safari Window 1965-79 Sedan / Triple Chrome Plating at the best online prices at eBay! Free shipping for many products!
Kit front Safari window for bug. Made in Brazil in carbon steel chrome. Kit: frame, glass, rubber seal, latches. I have 2 models: for bug (beetle) after 1963 with flat glass. for oval beetle It fits only for beetles up to 1959. For prices, payment, delivery time please contact me. Add a Comment. Sort by:
T1 1953-57 Beetle Chrome Front Pop-out Safari Window Frame Kit. £ 649.95. Beautifully produced by the team at Loose Nuts. We are proud to present these Front Safari Window Kits for T1 Beetle. SKU: 080070001.
On sale now: Safari Front Window Pop Out Kit, Standard Bug Sedan 1968-77, Super Beetle Sedan 1971-72, Chrome Frame, Sliders, Latches, Seals & Hardware *Made In Usa* ... However some people just screw them on but is not recommended if you plan on cruising at speed with the Safari window open. Details. SKU: 361-200; Stock #: 361-200;
Front Safari Window Bug Beetle 1958 64 and 1965 79 Price: $599. We are very glad to present our front Safari Window for Bugs from 1958 -1964 Includes the triple chrome plating external frame (same material used on the original pop out windows) and all other components also made of stainless steel. We do also produce this item for 1965-79 - FLAT ...
Bulk Buy: Buy 2 for £12.83 each. Add 2 to Boot. Buy 5 for £12.16 each. Add 5 to Boot. This part fits: **ON SALE** Splitscreen Bus Side Popout Window Seal (Glass To Frame Seal) Part Number: 221845325B. Glass to frame seal for popout side windows fitted to any year Splitscreen buses Quality: Good quality Quantity: Sold each Bundle Kit: There are...
The video will expand to take up as much space as is available in your Safari window. The moment you click away, the video automatically goes into picture-in-picture mode, according to the demo ...
On Sale T1 1965-72 Beetle Rear Pop-out Safari Window Frame with Glass Kit - View Full Range of Exterior, Safari Windows and Rear Safaris - 080070007 -
Safari 5.1.7 for Windows, released in 2010 and now outdated, was the last version made for Windows. If a website says your browser is out of date. If a website says that Safari is out of date even though you're already using the latest version of macOS, iOS, iPadOS, or visionOS, there could be an issue with the website. If you're sure that ...
Safari Window Kits & Parts; West Coast Metric - Safari Window Kits & Parts. West Coast Metric Inc. 310-325-0005; 800-247-3202; 24002 Frampton Ave Harbor City, CA 90710; 24002 Frampton Ave; Harbor City, CA 90710; Office Hours: Mon - Fri 9:00 - 5:00 PM PST. Will Call Parts Pickup: Mon - Fri 9:00 - 4:30 PM PST.
Systematic Entomology is a specialized journal that focuses on publishing research papers concerning insect systematics, phylogenetics, & integrative taxonomy.
volkswagen beetle safari window frame and glasses beetle 1959 - 64. Est. delivery Fri, Jun 21 - Mon, Jul 8 to 23917. See details. Breathe easy. Returns accepted. US $260.00Standard Shipping from outside US. See details. International shipment of items may be subject to customs processing and additional charges.
The longhorn beetle assemblage at Repolheiro represents about 60% of the species at TES , and the greatest richness and abundance of beetles found in this area can be explained by the heterogeneity and availability of resources such as wood. Wood is a crucial resource for longhorn beetles, used for food as well as nesting.
For the replacement component (bsim), we get that tree communities had a significantly higher value than beetles at the scales of β 2, β 3 and δ 1 (Z = 6.7491, 5.6329 and 3.8215 respectively; P ...
The biodiversity structure and habitat requirements of longhorn beetles (Cerambycidae) in floodplain forests of the western part of Saratov oblast were studied from 2011 to 2014. A total of 51 species of longhorn beetles has been identified. The largest subfamilies are Cerambycinae (19 species), Lepturinae (17 species), and Lamiinae (13 species). The specific communities include 34, 14, 11, 9 ...