Official websites use .gov
A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.

CDC Current Outbreak List
Infectious disease outbreaks currently being reported on by CDC. Listings include those outbreaks for which content is currently published on the CDC website.
Recent investigations reported on CDC.gov
- Cucumbers – Salmonella Outbreak Announced June 2024
- Backyard Poultry – Salmonella Outbreaks Announced May 2024
- Organic Walnuts – E coli Outbreak Announced April 2024
- Fresh Basil – Salmonella Outbreak Announced April 2024
- Measles Outbreaks 2024 Announced January 2024
- Coronavirus Disease 2019 (COVID-19) Announced January 2020
Please see the Travelers’ Health site for a complete list.
In the last two years, CDC has sent scientists and doctors out more than 750 times to respond to health threats. Learn more below.
- Investigating Foodborne Outbreaks
- Waterborne Outbreaks Toolkit
- Ebola Outbreak History Announced September 2022
- Mpox Outbreaks Announced May 2022
- Multistate Foodborne Outbreaks – Foodborne outbreaks listed by year
- Hepatitis A Outbreaks – Hepatitis A outbreak investigations since 2013 where CDC supported or led the investigation.
- US Outbreaks Linked to Contact with Animals or Animal Products
- Health Alert Network – Health alerts, health advisories, updates, and info service messages. Designed for public health and medical communities.
- Recent Outbreaks and Incidents – Events involving the CDC Emergency Operations Center
- Morbidity and Mortality Weekly Report – Outbreak investigation reports included among other content. Note that outbreak material includes state health department investigations. Designed for public health and medical communities.
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.
- Type 2 Diabetes
- Heart Disease
- Digestive Health
- Multiple Sclerosis
- Diet & Nutrition
- Supplements
- Health Insurance
- Public Health
- Patient Rights
- Caregivers & Loved Ones
- End of Life Concerns
- Health News
- Thyroid Test Analyzer
- Doctor Discussion Guides
- Hemoglobin A1c Test Analyzer
- Lipid Test Analyzer
- Complete Blood Count (CBC) Analyzer
- What to Buy
- Editorial Process
- Meet Our Medical Expert Board
CDC Updates COVID Travel Guidance for 120 Countries Ahead of Summer
Images By Tang Ming Tung / Getty Images
Key Takeaways
- The Centers for Disease Control and Prevention has updated its COVID travel guidance for more than a hundred countries.
- The CDC classifies travel to each country by level of COVID-19 risk.
- Infectious disease experts say it's relatively safe to travel internationally now, provided you're vaccinated and avoid high-risk countries.
The Centers for Disease Control and Prevention (CDC) has issued new COVID-19 travel guidelines for more than 120 countries.
The updated advice offers detailed information on specific countries for travelers who are vaccinated and for those who aren't. The CDC specifically assigns risk levels to each country based on the number of cases per 100,000 people.
To use the new guidance , travelers can search by the name of the country they’re interested in visiting and then learn whether its COVID-19 risk is very high, high, moderate, low, or unknown.
The CDC also offers detailed advice on what to do to stay safe in that particular country, broken down by whether you’re vaccinated or unvaccinated.
The new guidance arrives as COVID-19 cases dip to low levels previously seen at the start of the pandemic in March 2020. As vaccination increases and cases drop, travel among Americans is picking up speed.
Just days after the CDC's updated guidance, the Transportation Security Administration (TSA) announced that it screened more than 2 million passengers on the same day for the first time since March 2020.
What the Risk Levels Mean
The CDC divides up countries based on four levels of risk.
Level 4: Very High Risk
Countries that are classified as very high-risk have more than 500 new cases per 100,000 people over the past 28 days. The CDC recommends against traveling to these countries, which currently include Brazil, India, and Iraq.
Level 3: High Risk
These countries have 100 to 500 cases per 100,000 residents. The CDC specifically recommends avoiding nonessential travel to these countries—including Mexico, Russia, and Iran—for people who are unvaccinated.
Level 2: Moderate Risk
Level 2 countries have 50 to 99 cases per 100,000, and currently include Finland, Cambodia, and Kenya. The CDC suggests that people who are unvaccinated and at increased risk of severe illness from COVID-19 avoid these countries.
Level 1: Low Risk
Level 1 countries are nations with less than 50 COVID-19 cases per 100,000 people, and include Australia, Israel, and Singapore. The agency still recommends that you get vaccinated before you travel to one of them.
What This Means For You
The CDC breaks down which countries are considered high and low risk for travel right now. If you decide to travel internationally, first check the risk level of the country you’re interested in visiting, and don’t forget to wear a mask on your journey.
Is International Travel Safe Right Now?
Infectious disease experts say your safety depends on your vaccination status. “For a fully vaccinated individual, international travel is low-risk,” infectious disease expert Amesh A. Adalja, MD , senior scholar at the Johns Hopkins Center for Health Security, tells Verywell.
“If you’re fully vaccinated, I think international travel is OK,” Thomas Russo, MD , professor and chief of infectious disease at the University at Buffalo in New York, tells Verywell. “But one should be a little more cognizant of countries that have variants of concern, like India.”
While Russo says that “the consequences for fully vaccinated individuals might not be as bad” as they would be for those who aren't vaccinated, there is still a chance that they can bring the variants back home after traveling. “We’re trying to minimize importing those strains,” he says.
But there are “issues related to air travel in general,” Stanley Weiss, MD , professor of medicine at the Rutgers New Jersey Medical School, tells Verywell. “There is the issue of yourself and those near you, in screening areas, waiting areas, and on the plane."
Weiss points out that you can be exposed to COVID for long periods of time on international flights. And there's “a considerable period of time” where everyone may not always be masked.
Ultimately, “the decision to take an international trip currently is really a risk/benefit consideration that is dependent upon one's own risk tolerance,” Prathit Kulkarni, MD , assistant professor of medicine in infectious diseases at Baylor College of Medicine, tells Verywell.
He recommends being mindful of the risk level of the country you’re interested in visiting. “If it is possible to defer a trip to a country with very high transmission currently until transmission slows down a bit, that is likely preferred,” Kulkarni says.
It’s important to keep in mind that “you may not be able to get reasonable healthcare if you get sick” while visiting a higher risk area, John Sellick, DO , an infectious disease expert and professor of medicine at the University at Buffalo in New York, tells Verywell.
Adalja agrees. “Traveling to a very high-risk area, even when fully vaccinated, probably is not going to be a very fun trip if a country is spiraling out of control because of COVID-19 cases and hospitals in crisis,” he says.
How to Stay Safe When You Travel
“Sometimes there are extenuating circumstances necessitating travel; In this case, prevention steps are the best way to reduce risk,” Kulkarni says. That includes wearing a mask in the airport and on the plane and practicing good hand hygiene, Sellick adds.
Be aware, too, that you may have to get tested or quarantine—even if you’re fully vaccinated—because of regulations in place in other countries, Adalja says.
Weiss recommends bringing extra masks along for the trip and keeping a spare on you at all times. “You need enough with you to enable you to change them,” he says.
Overall, public health experts stress the importance of getting vaccinated before you travel. “It’s the most important thing you can do,” Russo says.
The information in this article is current as of the date listed, which means newer information may be available when you read this. For the most recent updates on COVID-19, visit our coronavirus news page .
Centers for Disease Control and Prevention. How CDC determines the level for COVID-19 travel health notices .
Centers for Disease Control and Prevention. COVID-19 travel recommendations .
Centers for Disease Control and Prevention. Trends in number of COVID-19 cases and deaths in the US reported to CDC, by state/territory .
Transportation Security Administration. TSA surpasses 2 million daily travelers screened .
By Korin Miller Miller is a health and lifestyle journalist with a master's degree in online journalism. Her work appears in The Washington Post, Prevention, SELF, Women's Health, and more.
Infectious diseases
On this page, when to see a doctor, risk factors, complications, infectious diseases care at mayo clinic.
Our caring teams of professionals offer expert care to people with infectious diseases, injuries and illnesses.
Infectious diseases are disorders caused by organisms — such as bacteria, viruses, fungi or parasites. Many organisms live in and on our bodies. They're normally harmless or even helpful. But under certain conditions, some organisms may cause disease.
Some infectious diseases can be passed from person to person. Some are transmitted by insects or other animals. And you may get others by consuming contaminated food or water or being exposed to organisms in the environment.
Signs and symptoms vary depending on the organism causing the infection, but often include fever and fatigue. Mild infections may respond to rest and home remedies, while some life-threatening infections may need hospitalization.
Many infectious diseases, such as measles and chickenpox, can be prevented by vaccines. Frequent and thorough hand-washing also helps protect you from most infectious diseases.
Products & Services
- A Book: Endemic - A Post-Pandemic Playbook
- A Book: Mayo Clinic Family Health Book, 5th Edition
- Newsletter: Mayo Clinic Health Letter — Digital Edition
Each infectious disease has its own specific signs and symptoms. General signs and symptoms common to a number of infectious diseases include:
- Muscle aches
Seek medical attention if you:
- Have been bitten by an animal
- Are having trouble breathing
- Have been coughing for more than a week
- Have severe headache with fever
- Experience a rash or swelling
- Have unexplained or prolonged fever
- Have sudden vision problems
From Mayo Clinic to your inbox
Infectious diseases can be caused by:
- Bacteria. These one-cell organisms are responsible for illnesses such as strep throat, urinary tract infections and tuberculosis.
- Viruses. Even smaller than bacteria, viruses cause a multitude of diseases ranging from the common cold to AIDS.
- Fungi. Many skin diseases, such as ringworm and athlete's foot, are caused by fungi. Other types of fungi can infect your lungs or nervous system.
- Parasites. Malaria is caused by a tiny parasite that is transmitted by a mosquito bite. Other parasites may be transmitted to humans from animal feces.
Direct contact
An easy way to catch most infectious diseases is by coming in contact with a person or an animal with the infection. Infectious diseases can be spread through direct contact such as:
Person to person. Infectious diseases commonly spread through the direct transfer of bacteria, viruses or other germs from one person to another. This can happen when an individual with the bacterium or virus touches, kisses, or coughs or sneezes on someone who isn't infected.
These germs can also spread through the exchange of body fluids from sexual contact. The person who passes the germ may have no symptoms of the disease, but may simply be a carrier.
- Animal to person. Being bitten or scratched by an infected animal — even a pet — can make you sick and, in extreme circumstances, can be fatal. Handling animal waste can be hazardous, too. For example, you can get a toxoplasmosis infection by scooping your cat's litter box.
- Mother to unborn child. A pregnant woman may pass germs that cause infectious diseases to her unborn baby. Some germs can pass through the placenta or through breast milk. Germs in the vagina can also be transmitted to the baby during birth.
Indirect contact
Disease-causing organisms also can be passed by indirect contact. Many germs can linger on an inanimate object, such as a tabletop, doorknob or faucet handle.
When you touch a doorknob handled by someone ill with the flu or a cold, for example, you can pick up the germs he or she left behind. If you then touch your eyes, mouth or nose before washing your hands, you may become infected.
Insect bites
Some germs rely on insect carriers — such as mosquitoes, fleas, lice or ticks — to move from host to host. These carriers are known as vectors. Mosquitoes can carry the malaria parasite or West Nile virus. Deer ticks may carry the bacterium that causes Lyme disease.
Food contamination
Disease-causing germs can also infect you through contaminated food and water. This mechanism of transmission allows germs to be spread to many people through a single source. Escherichia coli (E. coli), for example, is a bacterium present in or on certain foods — such as undercooked hamburger or unpasteurized fruit juice.
More Information
- Ebola transmission: Can Ebola spread through the air?
- Mayo Clinic Minute: What is the Asian longhorned tick?
While anyone can catch infectious diseases, you may be more likely to get sick if your immune system isn't working properly. This may occur if:
- You're taking steroids or other medications that suppress your immune system, such as anti-rejection drugs for a transplanted organ
- You have HIV or AIDS
- You have certain types of cancer or other disorders that affect your immune system
In addition, certain other medical conditions may predispose you to infection, including implanted medical devices, malnutrition and extremes of age, among others.
Most infectious diseases have only minor complications. But some infections — such as pneumonia, AIDS and meningitis — can become life-threatening. A few types of infections have been linked to a long-term increased risk of cancer:
- Human papillomavirus is linked to cervical cancer
- Helicobacter pylori is linked to stomach cancer and peptic ulcers
- Hepatitis B and C have been linked to liver cancer
In addition, some infectious diseases may become silent, only to appear again in the future — sometimes even decades later. For example, someone who's had chickenpox may develop shingles much later in life.
Follow these tips to decrease the risk of infection:
- Wash your hands. This is especially important before and after preparing food, before eating, and after using the toilet. And try not to touch your eyes, nose or mouth with your hands, as that's a common way germs enter the body.
- Get vaccinated. Vaccination can drastically reduce your chances of contracting many diseases. Make sure to keep up to date on your recommended vaccinations, as well as your children's.
- Stay home when ill. Don't go to work if you are vomiting, have diarrhea or have a fever. Don't send your child to school if he or she has these signs, either.
Prepare food safely. Keep counters and other kitchen surfaces clean when preparing meals. Cook foods to the proper temperature, using a food thermometer to check for doneness. For ground meats, that means at least 160 F (71 C); for poultry, 165 F (74 C); and for most other meats, at least 145 F (63 C).
Also promptly refrigerate leftovers — don't let cooked foods remain at room temperature for long periods of time.
- Practice safe sex. Always use condoms if you or your partner has a history of sexually transmitted infections or high-risk behavior.
- Don't share personal items. Use your own toothbrush, comb and razor. Avoid sharing drinking glasses or dining utensils.
- Travel wisely. If you're traveling out of the country, talk to your doctor about any special vaccinations — such as yellow fever, cholera, hepatitis A or B, or typhoid fever — you may need.
- Vaccine guidance from Mayo Clinic
- Enterovirus D68 and parechovirus: How can I protect my child?
- What are superbugs and how can I protect myself from infection?
Feb 18, 2022
- Facts about infectious disease. Infectious Disease Society of America. https://www.idsociety.org/public-health/facts-about-id/. Accessed May 29, 2019.
- Jameson JL, et al., eds. Approach to the patient with an infectious disease. In: Harrison's Principles of Internal Medicine. 20th ed. New York, N.Y.: The McGraw-Hill Companies; 2018. https://accessmedicine.mhmedical.com. Accessed May 29, 2019.
- Clean hands count for safe health care. Centers for Disease Control and Prevention. https://www.cdc.gov/features/handhygiene/index.html. Accessed May 29, 2019.
- Kumar P, et al., eds. Infectious diseases and tropical medicine. In: Kumar and Clark's Clinical Medicine. 11th ed. Philadelphia, Pa.: Elsevier; 2017. https://www.clinicalkey.com. Accessed May 29, 2019.
- LaRocque R, et al. Causes of infectious diarrhea and other foodborne illnesses in resource-rich settings. https://www.uptodate.com/contents/search. Accessed May 29, 2019.
- Ryan KJ, ed. Infectious diseases: Syndromes and etiologies. In: Sherris Medical Microbiology. 7th ed. New York, N.Y.: McGraw-Hill Education; 2018. https://accessmedicine.mhmedical.com. Accessed May 29, 2019.
- File TM, et al. Epidemiology, pathogenesis, and microbiology of community-acquired pneumonia in adults. https://www.uptodate.com/contents/search. Accessed May 29. 2019.
- DeClerq E, et al. Approved antiviral drugs over the past 50 years. Clinical Microbiology Reviews. 2016;29:695.
- Mousa HAL. Prevention and treatment of influenza, influenza-like illness and common cold by herbal, complementary, and natural therapies. Journal of Evidence-Based Complementary & Alternative Medicine. 2017;22:166.
- Caring for someone sick. Centers for Disease Control and Prevention. https://www.cdc.gov/flu/treatment/caring-for-someone.htm. Accessed May 29, 2019.
- Diseases & Conditions
- Infectious diseases symptoms & causes
News from Mayo Clinic

- Antibiotic use in agriculture
- Infection: Bacterial or viral?
- Monkeypox: What is it and how can it be prevented?
- Types of infectious agents
- What is chikungunya fever, and should I be worried?
CON-XXXXXXXX
We’re transforming healthcare
Make a gift now and help create new and better solutions for more than 1.3 million patients who turn to Mayo Clinic each year.

Search Smartraveller
Infectious diseases.

This page has information about the risk of infectious diseases for Australians overseas.
This page only gives general advice. Always talk to your healthcare providers for advice specific to you and your situation.
Read this page to learn about:
- infectious disease risks overseas
- vaccine-preventable diseases
- insect-borne diseases
- water or food-borne diseases
- contact, droplet or airborne diseases
- blood-borne diseases
- sexually transmissible infections (STIs)
- diseases spread by animals
- consular services and infectious diseases
Infectious disease risks overseas
Infectious diseases exist everywhere. But you have a higher risk of getting an infectious disease in some destinations. This can be due to:
- water and sanitation issues
- insects that carry diseases
- low vaccination rates.
Research the risks before you go. Then you can take preventive measures to reduce them.
- Read our travel advice for your destination . See if there are any health risks or suggested vaccines listed.
- Talk to your doctor or pharmacist. Tell them where you're going and ask their advice on prevention.
Vaccine-preventable diseases
Vaccination can prevent some diseases.
Get your travel vaccinations. Particularly if there's a travel warning for a vaccine-preventable disease where you're going. Ensuring you're vaccinated can protect you and those around you.
Talk to your doctor. Ask what travel vaccines they recommend. And find out if your standard vaccinations need boosting.
Get the vaccinations you need well before you go. Some take 6-8 weeks to work or need several doses.
Visit the Vaccine Hub to explore suggested vaccines for where you're going . Common travel vaccines include:
- chickenpox (varicella)
- hepatitis A and hepatitis B
- influenza (flu)
- Japanese encephalitis
- meningococcal
- tuberculosis (TB)
- yellow fever .
This list doesn't include everything. You may need to vaccinate against other diseases depending on your health and where you're going. Ask your doctor or pharmacist for advice.
Learn more about travel vaccinations .
Proof of vaccination
You may need proof of certain vaccinations when you're travelling.
Rules will vary between each country. Contact your destination's embassy or consulate in Australia for information.
Yellow fever
Some countries need you to show an international yellow fever vaccination certificate if you:
- arrive from a yellow fever-infected area
- transit through an infected area before arriving
- have been in an infected area within a number of days before arriving.
Your airline can stop you from boarding if you don't have one.
It's important to get your yellow fever vaccination at an approved clinic . You need it at least 10 days before you travel. The clinic will give you your certificate.
Read more about international yellow fever vaccination certificates .
Some countries may need you to show a COVID-19 vaccination certificate on entry or to access some services.
You can get your certificate through myGov or the Express Plus Medicare mobile app . If you can't use these options
- visit a Services Australia services centre
- contact the Australian Immunisation Register (AIR) at 1800 653 809 (in Australia) or +61 2 8633 3284 (from overseas).
Learn more about international COVID-19 vaccination certificates .
Insect-borne diseases
Insects can carry disease and spread it between people. They can also spread disease between animals and people.
You're more likely to get an insect-borne disease in tropical climates. This includes parts of
- Central America
- the Pacific.
You're also at risk in parts of Europe and the United States. Risks may differ depending on what you plan to do.
Some common insect-borne diseases are:
- chikungunya
- Lyme disease
- yellow fever
- Zika virus .
If you're pregnant or planning to get pregnant, your unborn child may face more risks from Zika virus. As a precaution, the Department of Health advises you to consider delaying travel to countries with Zika.
Reducing your risk of insect-borne diseases
You can get vaccinated against some insect-borne illnesses. For others, such as malaria, there's oral medicine you can take. Discuss options with your doctor or pharmacist.
Take steps to avoid insect bites.
- Cover exposed skin with light-coloured long-sleeved shirts and long pants.
- Use insect repellents with DEET or picaridin. If you use both sunscreen and insect repellent, apply the sunscreen first and then the repellent.
- Treat your clothing and gear with insecticides containing permethrin.
- Sleep in screened or air-conditioned rooms. Use bed nets if you can't keep mosquitoes from coming inside the room.
After walking through high grass or brush in tick-prone areas:
- brush off your clothes before getting in a car or going inside
- check yourself and others for ticks
- tumble dry your clothes hot for 20 minutes to kill ticks that may have hitched a ride.
Water- or food-borne diseases
Water- or food-borne diseases are common in many countries. You can catch them from:
- drinking or using tainted water
- drinking any drink with ice cubes made with tap water
- eating food washed with tap water
- brushing your teeth or washing your face with tap water
- eating tainted food, including meals prepared by people following poor hygiene practices
- taking part in activities in tainted water, such as swimming or water sports.
Some water- and food-borne diseases include:
- infections causing gastroenteritis (vomiting and diarrhoea )
- campylobacter
- hepatitis A
- leptospirosis
- polio (poliomyelitis)
- salmonella (including typhoid and paratyphoid )
- schistosomiasis
Cholera is less common, but it's very contagious and deadly.
Reduce your risk from water- or food-borne diseases
There are vaccines for some water and food-borne infectious diseases, including hepatitis A, typhoid and cholera.
You can do a lot to reduce your risk of infection where water and food-borne diseases are common.
- Wash your hands often and use hand sanitiser before you eat.
- Drink bottled water from a sealed bottle.
- Use water-sterilising tablets or filtration bottles to make tap water safe.
- Wash fresh produce thoroughly using bottled or sterilised water.
Try to avoid:
- drinking tap water or using it to clean your teeth
- drinks with ice cubes. Some restaurants may use water straight from the tap
- uncooked and undercooked food or food that has been left sitting for a while
- fresh salads and raw vegetables
- contact with water or soil that may be contaminated with sewerage
- in some places, swimming or wading in any fresh water.
For more information, see the Better Health Channel's advice on food safety while travelling .
Contact, droplet and airborne diseases
Some diseases can spread:
- through close contact with a person who is coughing, sneezing, or unwell
- through something an infected person touched after coughing, sneezing or blowing their nose.
These are called diseases spread by contact, droplets or through the air.
These include:
- chickenpox (varicella)
- coughs and colds
- influenza (flu)
- Middle East respiratory syndrome (MERS)
- Severe acute respiratory syndrome (SARS)
Reducing your risk of contact, droplet and airborne diseases
Protect yourself from contact, droplet and airborne diseases.
- Stay up to date with your vaccinations for measles, rubella, diphtheria, pertussis, flu, COVID-19, and other vaccines recommended by your doctor.
- Wash your hands often or use hand sanitiser if soap and water aren't available.
- Avoid close contact with people who are sick or showing early symptoms.
- Avoid close contact with wild or domestic animals.
Blood-borne diseases
Some infectious diseases can be spread by blood. This can be:
- when an infected person's blood comes into direct contact with another person's blood, mouth or eyes
- when blood is on an object such as a needle or razor blade
- through bodily fluids (see also sexually transmissible infections )
Common blood-borne diseases overseas are:
- hepatitis B
- hepatitis C
Reduce your risk of blood-borne diseases
You can get vaccinated against some blood-borne diseases.
You can also reduce your risk of infection by being careful and making smart choices.
- Avoid contact with any objects that could be tainted with blood or body fluids.
- Never share needles, syringes, or other injecting tools.
- Choose tattoo, piercing and medical services that carefully sterilise their tools and surfaces. If you're not sure, ask for proof before they start.
- Practise safer sex.
- Cover wounds and cuts with a waterproof dressing.
- Don't share toothbrushes, razors and other personal items that may be tainted with blood.
- Use gloves and other relevant protection when giving first aid.
These diseases may not have symptoms in the early stages. If you think you've been exposed, seek medical advice early. There may be preventive treatment.
Sexually transmissible infections (STIs)
STIs are infections or diseases passed on during unprotected sex with an infected partner, including vaginal, anal and oral sex. Some STIs can spread through skin-to-skin contact with a person's infected genitals or mouth.
STIs include:
- genital herpes
- gonorrhoea
- human papillomavirus (HPV)
Other diseases can spread through sexual contact but are not called an STI. For example, water-borne diseases shigellosis and hepatitis A can also spread through sexual activity, including oral and anal sex.
Reducing your risk of STIs
Vaccines can prevent some STIs. But you must also take preventive measures to reduce your risk of infection.
Reduce your risk of infection by taking and using your own condoms from a brand you trust.
STIs may not have symptoms. If you think you've been exposed, seek medical advice.
If you're a victim of assault , including sexual assault , get medical care immediately to reduce the risk of infection.
For more information, see the Department of Health's information on STIs and HIV / AIDS .
Diseases spread by animals
Some animals can spread disease through close contact, scratches or bites. These diseases include:
- influenza A subtypes , including avian and swine influenza
- Middle East respiratory syndrome (MERS)
Reduce your risk of infection.
- Get vaccinated for rabies and influenza before you go, if recommended.
- Avoid contact with sick animals.
- Wash your hands well after contact with animals.
- Don't eat raw or undercooked meat or animal products.
If you have a pre-existing illness, you may need to avoid all contact with some animals. Seek medical advice.
If you're bitten, scratched or licked on an open wound by an animal:
- use first aid
- wash the wound out well with soap and water for at least 10 minutes
- use antiseptic solution
- get urgent medical advice about preventing rabies, tetanus, and bacterial infection.
Consular services and infectious diseases
You're responsible for protecting yourself against infectious diseases.
Read the Consular Services Charter for what the Australian Government can and can't do to help you overseas .
What we can do
- We can update our travel advice if we learn of a disease outbreak.
- We can give you a list of local English-speaking doctors and health services.
- We can help you get in contact with family and friends in Australia.
- We can provide emergency support if we declare a disease outbreak a crisis.
What we can't do
- We can't tell you every infectious disease in your destination.
- We can't give you medical advice.
- We can't tell you all the vaccines you may need.
- We can't tell you what to do or take to protect your health in your destination.
- We can't guarantee your health in any destination.
- We can't pay your medical costs if you get sick overseas.
- Read about travelling with medication and medical equipment .
- See our advice on travelling while pregnant .
- Learn about medical tourism .
- See our health advice for everyone .
- Read advice about travelling with children .
- See our advice on taking care of your mental health .
- See our advice for travellers with a disability .
- Read about health risks in all your destinations .
- Understand how and when consular services can help Australians overseas.
- Learn about infectious diseases and travel vaccinations (Department of Health).
- See a range of advice on international travel and health (World Health Organization).
- Read advice on food safety while travelling (Victorian Department of Health and Human Services).
- Learn about immunising your child (Department of Health).
- See information about insect-borne diseases (World Health Organisation).
Related content
Read our general advice for Australians planning to travel overseas with medications or medical equipment.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Elsevier - PMC COVID-19 Collection

Human Mobility and the Global Spread of Infectious Diseases: A Focus on Air Travel
Aidan findlater.
1 Department of Medicine, University of Toronto, Toronto, Canada
Isaac I. Bogoch
2 Divisions of General Internal Medicine and Infectious Diseases, Toronto General Hospital, University Health Network, Toronto, Canada
Greater human mobility, largely driven by air travel, is leading to an increase in the frequency and reach of infectious disease epidemics. Air travel can rapidly connect any two points on the planet, and this has the potential to cause swift and broad dissemination of emerging and re-emerging infectious diseases that may pose a threat to global health security. Investments to strengthen surveillance, build robust early-warning systems, improve predictive models, and coordinate public health responses may help to prevent, detect, and respond to new infectious disease epidemics.
The volume of global air travel continues to increase annually, and passengers have the capacity to introduce infections to new regions in short time frames.
Infections transported through air travel may initiate or facilitate epidemics.
Front-line healthcare providers and public health teams require training and tools to properly identify and respond to infectious diseases transported through air travel, some of which may have epidemic potential.
Developing more effective global surveillance tools and mechanisms to better communicate and coordinate between countries can facilitate more rapid and effective responses to epidemics.
Human Mobility and the Spread of Infectious Diseases
Increases in the global mobility of humans, nonhuman animals, plants, and products are driving the introduction of infectious diseases to new locations. In recent years we have witnessed several infectious diseases spread well beyond their previously understood geographic boundaries, as was demonstrated by the introduction of Zika virus to the Americas. We have also witnessed the emergence and spread of novel pathogens, such as the discovery of a previously unknown Middle Eastern respiratory syndrome coronavirus (MERS-CoV) in Saudi Arabia spreading to distant countries such as South Korea [1] .
Many factors contribute to the global spread of infectious diseases, including the increasing speed and reach of human mobility, increasing volumes of trade and tourism, and changing geographic distributions of disease vectors. In particular, human travel and migration (especially via air travel) is now a major driving force pushing infections into previously nonendemic settings. Year by year, there are increasing numbers of international tourists [2] , more international refugees and migrants [3] , greater capacity for shipping by sea [4] , and greater international air travel passenger volumes [5] . Air travel poses a growing threat to global health security, as it is now possible for a traveler harboring an infection in one location on earth to travel to virtually any other point on the planet in only 1–2 days. Infections introduced via travel may be sporadic and have little potential for further transmission, such as Lassa fever introduced into European settings [6] . In other situations, infections introduced by air travel may cause self-limited local epidemics such as Chikungunya virus in Italy [7] . More recently, there are a growing number of examples of infections introduced to a new region that ultimately become endemic, such as Chikungunya virus in Latin America and the Caribbean [8] .
Vector-borne infections, including arthropod-borne viruses (arboviruses) present unique challenges as disease vectors such as mosquitoes can be carried overland, in boats, or on planes, and may travel between any two points on the globe within their lifespan. Such vectors have the potential to infect nontravelers in their new destination, as is seen in airport malaria [6] . Even if a vector is not infected, that vector may become endemic to a new region if there are suitable environmental conditions, and then potentially enable future epidemics. The most well-known example of this is the now-global distribution of Aedes aegypti and Ae. albopictus mosquitoes, the vectors responsible for a number of arbovirus infections including dengue, Chikungunya, and Zika viruses [9] . Vector introduction is further facilitated by ecological factors, such as climate change and urbanization, that may enable vectors to flourish in new regions.
Clinical and public health care providers must be aware of the fluid boundaries of infectious diseases and be cognizant of the potential for imported infections. Front-line healthcare providers must now have knowledge of an increasingly broad spectrum of emerging illnesses from around the world, and public health teams must be prepared to respond to individual cases that have epidemic potential (e.g., Ebola virus), and coordinate responses at local, national, and international levels. Preparation for planning mass gatherings, such as large sporting events or an annual religious pilgrimage, require special consideration for the potential of these events to contribute to global outbreaks. Here, we outline emerging and re-emerging infectious diseases that are spread via human mobility, with a focus on air travel. We discuss sporadic cases and localized epidemics, international epidemics, and then focus on clinical and public health implications.
Air Travel Contributing to Sporadic Travel-Related Infections of Epidemic Potential, and to Localized Epidemics
Travel-related illnesses are common and mostly mild and self-limited, such as traveler’s diarrhea. However, the list of infections imported by returned travelers is growing [10] , and many are capable of causing local epidemics ( Table 1 ).
Recent Emerging and Re-emerging Infectious Diseases of Global Health Significance, Whose Spread Was Facilitated by Air Travel
Despite a global push for eradication, malaria continues to cause significant global morbidity and mortality. This protozoal vector-borne infection is transmitted to humans by the bite of infected Anopheles mosquitoes that are found in many tropical and temperate countries [11] . Malaria is a common cause of febrile illness in returned travelers [12] , and delays in diagnosis may lead to poor patient outcomes, including death [13] . The incubation period varies from weeks to more than a month depending on the species, which frequently contributes to the delayed diagnosis of imported cases.
Airport malaria, where non-travelers near airports are infected by mosquitoes that have been imported by air travel [14] , is a challenging diagnosis as local clinicians may not suspect this infection. Although the spraying of arriving planes has reduced the incidence of cases, it continues to be a concern as volumes of travel continue to increase [15] and case reports of malaria in non-travelers continue to grow [16] . Regions that have eradicated malaria but still have Anopheles vectors present are at risk of malaria reintroduction, for example in Sri Lanka [17] .
The global distribution of drug-resistant malaria is also changing, and strains of multidrug-resistant Plasmodium falciparum appear to be spreading [18] . Although multidrug-resistant strains are still generally rare, there is growing concern that such strains may expand beyond their current boundaries through boat and air travel, heightening the need for better surveillance.
Schistosomiasis
Chronic infection with schistosomiasis is associated with gastrointestinal or urogenital pathology [19] . Schistosomiasis is acquired via contact with contaminated fresh water and requires specific snail intermediate hosts for disease transmission [19] . Locally acquired cases of schistosomiasis were discovered recently in France, and these were most certainly introduced by travelers from endemic settings in Africa [20] . Snails local to Corsica were found to be competent hosts of Schistosoma haematobium , Schistosoma bovis , and hybrids of the two infections. There is the potential for further disease expansion given ongoing human travel and migration from schistosomiasis-endemic regions to nonendemic areas with the requisite intermediate snail hosts.
Air Travel Contributing to International Epidemics and Pandemics
Air travel has contributed to several epidemics of global health significance in recent years. Typically, an infected individual, either symptomatic or within an incubation period, flies to a distant location and introduces this infection to the local population. Below, we discuss major international epidemics relevant to air travel after 2000.
Severe Acute Respiratory Syndrome (SARS)
The severe acute respiratory syndrome (SARS) outbreak of 2002–2003 is an example of an emerging infection causing several simultaneous epidemics in noncontiguous geographic regions. SARS is a respiratory tract infection caused by a zoonotic coronavirus (SARS-CoV), and is thought to have arisen in horseshoe bats and subsequently transmitted to humans through civets as intermediate hosts [21] .
The 2002 SARS epidemic originated in southern China and spread via air travel to 29 countries, causing local epidemics in Hong Kong, Taiwan, Canada, Singapore, Vietnam, and the Philippines [22] . The global epidemic lasted about 8 months, with 8096 probable cases and 774 deaths for a case-fatality rate of 10% [22] . SARS caused widespread panic and cost an estimated US$11 billion worldwide [23] . Although the public health response was swift and ultimately effective, this epidemic highlighted the need for increased international cooperation in the era of rapid global transit. This epidemic spurred revisions to the International Health Regulations (IHR) and introduced the most significant changes since their adoption [24] .
Middle East Respiratory Syndrome (MERS)
Another novel zoonotic respiratory coronavirus, MERS-CoV, originated in Saudi Arabia in 2012 [25] . Its reservoir is thought to be the dromedary camel. Similar to SARS-CoV, MERS-CoV causes a respiratory infection, though with a higher case-fatality rate of about 35% [26] .
Since 2012, over 2000 cases have been detected in 27 countries. South Korea experienced the largest epidemic outside of Saudi Arabia, which was initiated by a single infected business traveler returning from that country. This one index case at a single hospital resulted in an additional 184 confirmed cases at 17 hospitals, and caused 33 deaths before the epidemic ended 2 months later [27] . Infection risk appeared to be primarily through hospital-based contact and fomites rather than through household contacts [28] .
Although the virus is thought to have low epidemic potential, epidemics such as the one in South Korea are not unexpected [29] . There are a number of countries at risk of importing cases via air travel due to close trade and tourism ties to Saudi Arabia [30] . Additionally, the annual Hajj pilgrimage brings millions of pilgrims from around the world to Saudi Arabia, raising the potential for future epidemics [31] .
Ebola Virus
The 2014 Ebola virus disease (EVD) epidemic in West Africa also highlighted the risks of global infectious disease transmission in an increasingly connected world. This zoonotic virus has a probable bat reservoir [32] and is spread between humans through contact with infected body fluids. EVD can incubate for up to 3 weeks before presenting as a severe febrile illness with multisystem organ failure and hemorrhagic tendencies. Epidemics have largely been confined to Africa, with case fatality rates between 30% to upwards of 80% [33] .
The largest EVD epidemic began in Sierra Leone in 2014. Starting in a rural town, the epidemic spread via land travel to bordering Guinea and Liberia. From there, cases began appearing on several continents via international air travel. In the USA, for instance, one returned traveler infected two healthcare workers before local transmission stopped [34] . Similar imported cases were seen in Italy and the United Kingdom, and a short chain of healthcare-related transmission was documented following the return of an infected Spanish citizen back to Spain 35 , 36 . An infected individual flew to Nigeria and initiated a larger epidemic, with 19 confirmed cases in two cities resulting in seven deaths [37] . Ultimately, the Nigerian epidemic was halted by the heroic efforts of local public health teams [38] . By the time the West African EVD outbreak ended in 2016, there were 21 868 reported cases and 11 310 deaths, with imported cases in seven countries.
During the 2014 EVD epidemic, many countries hastily instituted policies that limited travel to and from EVD-affected countries. Some countries closed land and air borders with Guinea, Liberia, and Sierra Leone 39 , 40 , and others, including Australia and Canada, temporarily refused to issue visas to travelers from affected countries [41] , a policy not aligned with the World Health Organization (WHO)’s IHR. The USA imposed enhanced screening procedures that measured the temperature of returned travelers from affected countries for up to 3 weeks. The 2014 EVD epidemic highlighted how governments may rapidly impose policy related to an emerging infection of epidemic potential, although the effectiveness of many of these policies is still debated.
Influenza A virus causes predictable seasonal epidemics in both northern and southern hemispheres. Influenza A virus also circulates in birds and pigs and has the potential to mutate more rapidly compared to influenza B virus, allowing the virus to recombine with different strains and cause epidemics. Many individuals infected with influenza A virus will experience a self-limited febrile illness punctuated with myalgia and malaise, and this infection is also associated with severe illness and is responsible for roughly 300 000–600 000 deaths per year, globally [42] .
The 1918 influenza pandemic demonstrated the ability of influenza A virus to cause a global catastrophe in the era of increasing global mobility, as it resulted in an estimated 50 million deaths worldwide [43] . Seasonal influenza strains are now more mobile than ever, and the effect of air travel is a well-established mechanism for facilitating global influenza transmission 44 , 45 , 46 , 47 . The 2009 H1N1 influenza pandemic demonstrated the potential for global air travel networks to rapidly disseminate a novel influenza virus that emerged on a pig farm in Mexico and spread to the rest of the world 48 , 49 , resulting in approximately 123 000–203 000 deaths, globally [50] .
Air Travel Contributing to Infections with New Areas of Endemicity
Human mobility continues to introduce infections to new geographic locations, and air travel contributes to this process. Occasionally, infections introduced to new regions may become endemic.
Aedes spp. Mosquitoes
The global emergence of arboviruses, such as dengue, Zika, and Chikungunya viruses, demonstrates how certain infections may become endemic in new regions if they are imported to areas with suitable ecological conditions. These arboviruses require Ae. aegypti or Ae. albopictus mosquito vectors for transmission, and at least one of these species of mosquito is now present on every continent except Antarctica [9] . Ae. albopictus , the Asian tiger mosquito, is well adapted to urban environments [51] and has contributed to recent arboviral epidemics. These mosquito vectors have spread along human trade and travel routes 51 , 52 , and diseases carried by such vectors are quickly following the same path.
Dengue Virus
Dengue virus, transmitted primarily by Ae. aegypti , is one of the most important re-emerging infectious diseases, with an estimated 2.5 billion people living in regions suitable for infection [53] . Dengue virus has four serotypes, each of which circulates independently, causing outbreaks in populations that are naïve to that serotype [54] . Though once well controlled, dengue virus re-emerged in Asia in the 1950s, and is now endemic or epidemic in many tropical and subtropical regions around the world. Its re-emergence over the past half-century has been driven by urbanization, globalization, inadequate vector control [55] , and, notably, increasing human travel 56 , 57 . Autochthonous transmission has now been described in the USA, France, Croatia, and Madeira 58 , 59 , 60 , all imported by infected travelers. Air travel is contributing to the increasing frequency of epidemics as new serotypes are introduced into susceptible populations by travelers 61 , 62 . Interestingly, there is emerging evidence that individuals infected with dengue virus who are asymptomatic or presymptomatic (and presumably have lower levels of viremia) are still able to infect competent mosquito vectors. Such individuals may be more prone to traveling given their lack of symptoms, and are likely contributing to global dengue virus transmission 63 , 64 .
Chikungunya Virus
Chikungunya virus is another arbovirus that is transmitted by Ae. aegypti and Ae. albopictus mosquito vectors. It causes a self-limited febrile illness with a syndrome of arthralgia or arthritis that may last for weeks to months after infection. Chikungunya virus was originally endemic in African and Asian settings, but has caused localized epidemics in Italy in 2007, and in Italy and France in 2017 7 , 60 , 65 , 66 . The 2007 epidemic in Italy was initiated by a single viremic traveler returning from India [7] , highlighting the role of air travel in its spread. In December of 2013, autochthonous cases of Chikungunya infection appeared in Saint Martin, also likely introduced by a viremic traveler. Given the appropriate ecological suitability and competent vectors for transmission, Chikungunya virus rapidly spread throughout Latin America and the Caribbean, affecting 46 countries and over 3 million people, and is now endemic in the Americas [67] . An evaluation of global travel patterns from endemic locations predicted that imported cases of Chikungunya would be common in New York, Miami, and Puerto Rico, with autochthonous transmission in the latter two regions [8] , which was ultimately realized 1 year later [68] .
Zika virus is also transmitted by Ae. aegypti and Ae. albopictus vectors and typically causes a self-limited febrile illness in approximately 20% of those affected [69] . The virus has a predilection for developing nervous systems, and microcephaly is well documented in children born to infected pregnant mothers [70] . Zika virus originated in Africa and spread slowly eastward to Asia during the second half of the 20th century, culminating in a large outbreak on the Yap island in 2007 [69] . This was followed by a number of smaller outbreaks in Pacific islands, eventually resulting in an unprecedented epidemic in Brazil in 2015 that spread through much of Latin America and the Caribbean [71] . Although the index case was not identified, molecular clock analyses suggest that the virus was introduced multiple times in 2013 or 2014 from viremic travelers [71] . The virus was accurately predicted to spread throughout Latin America and the Caribbean based on human air travel patterns from Brazil, to regions with suitable ecological conditions and competent mosquito vectors for disease transmission [72] . Similarly, Zika virus is predicted to be reintroduced to African and Asia-Pacific nations based on current air travel patterns 73 , 74 . It is currently unclear if Zika virus will be established as an endemic infection in Latin American and Caribbean nations, though there are several parallels with Chikungunya virus in this region, suggesting that this is a likely scenario [75] .
Antimicrobial Resistance
The discovery of antibiotics heralded a new era in medicine, but was almost immediately followed by the emergence of drug resistance, such as the early descriptions of penicillin resistance in Staphylococcus aureus species [76] . Treatment of organisms harboring antimicrobial resistance (AMR) genes is associated with treatment failures, worse clinical outcomes, and greater expense [77] . The devastating economic and health impacts of AMR are expected to be amplified in low-income countries [78] . The rapid rise of broad-spectrum AMR among Gram-negative bacteria, especially the Enterobacteraciae, is a growing public health threat. Soon after the development of extended-spectrum cephalosporins in the 1980s there emerged Enterobacteraciae with extended-spectrum beta-lactamases (ESBLs) and AmpC cephalosporinases [79] . These resistance genes were soon found worldwide in a variety of Gram-negative organisms, including Klebsiella , Escherichia coli , Salmonella , and others [80] . Carbapenemases are now increasingly commonplace, conferring resistance to some of the broadest-spectrum antibiotics [81] .
International air travel is contributing to this global spread of resistant organisms. Travelers returning from areas with a high prevalence of AMR often return colonized with resistant organisms, and these individuals can transmit AMR organisms to others [82] . The New Delhi metallo-beta-lactamase-1 (NDM-1) gene is a recent example of the potential for AMR to spread rapidly. Organisms with NDM-1 are resistant to essentially all cephalosporins and carbapenem antibiotic classes [83] . The first case report of NDM-1 was in a traveler who acquired a urinary infection while in India in 2008 [84] . By 2010, NDM-1 was detected in the USA, Canada, Japan, Kenya, Oman, Australia, the United Kingdom, and multiple other European countries [85] . Similarly, colistin resistance with the mobilized colistin resistance-1 ( mcr-1) gene is a very concerning development. From its likely origin in China, mcr-1 has been detected in returned travelers around the world 86 , 87 . Like NDM-1, it can colonize travelers returning from endemic areas [88] , and local transmission is now being seen outside of China [89] . These bacteria are emerging far from their site of origin largely due to human travel and agricultural trade. The WHO has recognized AMR as a major global health threat and has enacted a Global Action Plan to help combat its spread [90] . However, large-scale antimicrobial stewardship programs are currently not realized in much of the world.
Clinical Implications of Infections Transmitted via Air Travel
Given the increases in global tourism and human migration, physicians and other healthcare workers are seeing more travel-related infections. Since infections from distant locales can present to virtually any clinic or hospital in the world in a matter of days, healthcare workers must be aware of both local and international epidemics. For example, the index case of MERS in Korea had visited four healthcare facilities over 8 days before a diagnosis was established [91] , and this delayed diagnosis contributed to the epidemic. The ability of a clinician to obtain a quality travel history is increasingly important, even for routine infectious syndromes like an upper respiratory tract infection. Several tools now exist for clinicians to help keep abreast of distant epidemics that may be relevant to their clinical practice, such as ProMED-Mail [92] and HealthMap [93] . However, questions still remain about the best way to consume and process a large volume of data for which there may only be limited pertinent information.
It is also important for clinicians to ask about a more remote travel history. For example, tuberculosis testing is usually considered before starting immunosuppressive medications. However, asking about potential exposures to parasitic infections, such as Strongyloides stercoralis , is frequently forgotten. Strongyloides infections can disseminate following immunosuppression and cause a severe and frequently fatal disease [94] . Screening for Strongyloides is now recommended prior to initiating immunosuppressive medications in patients who have spent significant time in endemic countries [95] . Similarly, neurocysticercosis should be considered in those with seizure disorders from endemic countries, even if they have not visited an endemic region in many years. The differential diagnosis for common syndromes often expands when a lifetime travel history is considered, and this information has potential to significantly impact clinical decision-making.
Public Health Implications of Infections Transmitted via Air Travel
Epidemics such as those caused by SARS, MERS, and Ebola viruses highlight the importance of rapid public health responses to emerging epidemics in the era of expanding global air travel. Public health agencies at local and international levels must be aware of global outbreaks and emerging threats, and have the tools to initiate a rapid and coordinated response. Several systems need to be in place to facilitate such coordinated responses, including surveillance tools and methods of communication and management in various jurisdictions.
The revised IHR adopted after the 2002 SARS outbreak was designed to improve global reporting of outbreaks [24] and this document continues to function as an effective data-sharing agreement. While SARS and the revised IHR have resulted in an increased investment in surveillance among higher-income countries, countries with fewer resources but a higher burden of infectious disease epidemics have ongoing challenges operating surveillance programs. Improving global surveillance capacity is a priority, and countries with limited public health capacity may require logistic and infrastructure support for these global systems to function effectively 96 , 97 . In addition to disease surveillance networks, early-warning systems are increasingly helpful for monitoring the global spread of infectious diseases. Such systems utilize multiple data sources, including voluntary reports, international news, and social media 92 , 93 , 98 , 99 . Other systems, such as GeoSentinel and TropNet, coordinate tropical and travel medicine clinics that collect data on illnesses in returned travelers. These travelers essentially act as sentinels of emerging epidemics when a source country does not have the capacity to detect these infections in a timely manner. Such networks have detected a sarcocystis outbreak in Malaysia [100] , dengue virus outbreaks in Angola [101] , and tracked the spread of Zika virus globally [102] . With improved surveillance and early warning systems, and by harnessing travelers as sentinels, it is possible to piece together a global picture of emerging and re-emerging infectious diseases. Responding to these epidemics, though, still poses several challenges.
Travel-related policy and public health responses to epidemics take several approaches, and include travel restrictions to and from affected areas, and passenger screening. Travel bans and restrictions are generally not helpful in halting the spread of infectious diseases 45 , 103 , 104 , 105 , 106 . Such policies also may hinder affected countries coping with the epidemic and can impose significant economic burden above the direct costs of the infectious outbreak [107] . Travel restrictions also violate the revised IHR, effectively punishing countries for participating in global disease surveillance and discouraging open communication [108] . Screening of travelers from affected counties also poses logistical problems. Screening commonly relies on either self-reported symptoms (such as an influenza-like illness), which may be inaccurate, or on periodic body temperature screening such as using thermometers or thermal imaging technology. Travelers may be completely asymptomatic if they are in an incubation period during the time of travel, and this may be especially pertinent for infections with longer incubation periods such as Ebola virus [109] . Ultimately, resources may be better channeled to controlling the epidemic at the source.
Travel-Related Infectious Diseases in Low-Income and Lower-Middle-Income Countries
The burden of infectious diseases is disproportionately high in low-income and lower-middle-income countries (LICs and LMICs, respectively) [110] . Improving disease surveillance capabilities, vector control initiatives, developing laboratory capacity 111 , 112 , 113 , and supporting public health interventions such as vaccination, is essential to mitigate local impact and future dissemination of epidemics [114] . If an epidemic of global health significance is detected in an LIC or LMIC, communication and coordination with local public health teams is essential, as is supporting the capacity of local laboratories and personnel. This was recently helpful in halting the 2018 Madagascar plague epidemic 115 , 116 .
Concluding Remarks
The capacity for an infected human to rapidly travel between any two points on earth has heralded a new era in global health security as infectious diseases are able to spread more effectively than at any other time in history. The global public health response must be proportional.
Quality surveillance, open communication, and global coordination are key elements to prevent, detect, and extinguish epidemics early. Similarly, vaccine development and vector-control efforts may proactively prevent the emergence of epidemics. New tools are needed to enable front-line healthcare workers to diagnose non-local infections, as well as to facilitate rapid data sharing during outbreaks (see Outstanding Questions). Investing in capacity building targeted at detecting and responding to epidemics in LICs and LMICs is likely to be a very effective and cost-effective mode of preventing disease transmission worldwide.
Outstanding Questions
How do we most effectively harness the current global infectious disease surveillance capacity given the existing gaps?
Can stronger modeling more accurately predict where infections will spread through air travel, and can this determine what the next epidemic of global health significance will be?
How can we enable local healthcare workers to more effectively utilize global infectious diseases surveillance data?
How do we develop better methods to screen for passengers harboring infectious diseases prior to traveling, or after arrival?
How can international and local travel-related policies be strengthened to more effectively respond to infectious diseases of epidemic potential?
What is the best way to support low-income and lower-middle-income countries responding to infections with epidemic potential?
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 13 October 2021
Infectious disease in an era of global change
- Rachel E. Baker ORCID: orcid.org/0000-0002-2661-8103 1 , 2 ,
- Ayesha S. Mahmud 3 ,
- Ian F. Miller ORCID: orcid.org/0000-0002-2673-9618 1 , 4 ,
- Malavika Rajeev 1 ,
- Fidisoa Rasambainarivo 1 , 2 , 5 ,
- Benjamin L. Rice 1 , 6 ,
- Saki Takahashi 7 ,
- Andrew J. Tatem 8 ,
- Caroline E. Wagner 9 ,
- Lin-Fa Wang ORCID: orcid.org/0000-0003-2752-0535 10 , 11 ,
- Amy Wesolowski 12 &
- C. Jessica E. Metcalf 1 , 13
Nature Reviews Microbiology volume 20 , pages 193–205 ( 2022 ) Cite this article
166k Accesses
443 Citations
591 Altmetric
Metrics details
- Infectious diseases
- Policy and public health in microbiology
The twenty-first century has witnessed a wave of severe infectious disease outbreaks, not least the COVID-19 pandemic, which has had a devastating impact on lives and livelihoods around the globe. The 2003 severe acute respiratory syndrome coronavirus outbreak, the 2009 swine flu pandemic, the 2012 Middle East respiratory syndrome coronavirus outbreak, the 2013–2016 Ebola virus disease epidemic in West Africa and the 2015 Zika virus disease epidemic all resulted in substantial morbidity and mortality while spreading across borders to infect people in multiple countries. At the same time, the past few decades have ushered in an unprecedented era of technological, demographic and climatic change: airline flights have doubled since 2000, since 2007 more people live in urban areas than rural areas, population numbers continue to climb and climate change presents an escalating threat to society. In this Review, we consider the extent to which these recent global changes have increased the risk of infectious disease outbreaks, even as improved sanitation and access to health care have resulted in considerable progress worldwide.

Similar content being viewed by others

Emerging and re-emerging pediatric viral diseases: a continuing global challenge
Epidemiological and clinical characteristics of the COVID-19 epidemic in Brazil

Temporal shifts in 24 notifiable infectious diseases in China before and during the COVID-19 pandemic
Introduction.
In premodern times, colonization, slavery and war led to the global spread of infectious diseases, with devastating consequences (Fig. 1a ). Human diseases such as tuberculosis, polio, smallpox and diphtheria circulated widely, and before the advent of vaccines, these diseases caused substantial morbidity and mortality. At the same time, animal diseases such as rinderpest spread along trade routes and with travelling armies, with devastating impacts on livestock and dependent human populations 1 . However, in the past two decades, medical advances, access to health care and improved sanitation have reduced the overall mortality and morbidity linked to infectious diseases, particularly for lower respiratory tract infections and diarrhoeal disease (Fig. 1d ). The swift development of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine speaks to the efficacy of modern science in rapidly countering threats from emerging pathogens. Nevertheless, infectious disease burden remains substantial in countries with low and lower-middle incomes, while mortality and morbidity associated with neglected tropical diseases, HIV infection, tuberculosis and malaria remain high. Moreover, deaths from emerging and re-emerging infections, in comparison with seasonal and endemic infections, have persisted throughout the twenty-first century (Fig. 1c ). This points to a possible new era of infectious disease, defined by outbreaks of emerging, re-emerging and endemic pathogens that spread quickly, aided by global connectivity and shifted ranges owing to climate change (Fig. 1d ).
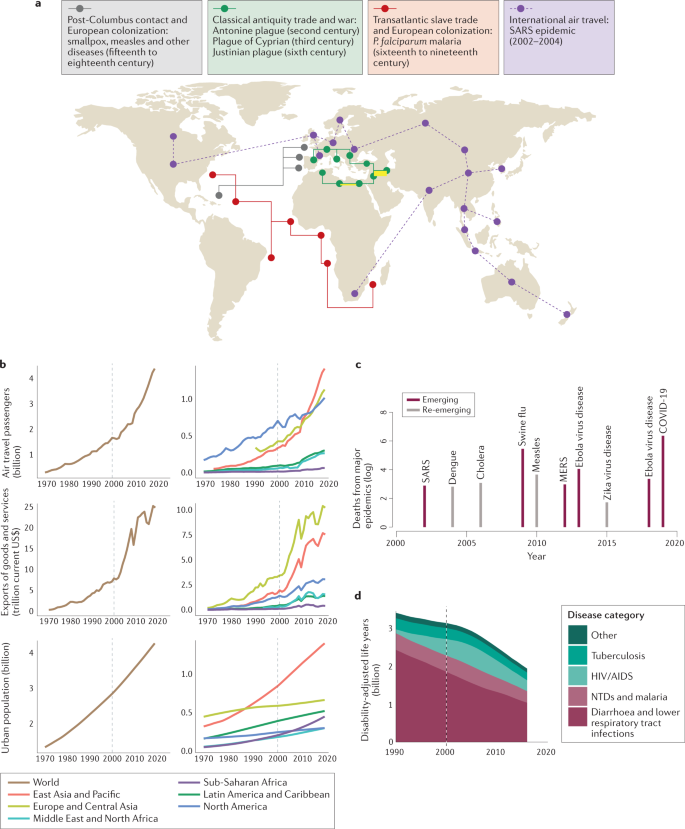
a | Examples of epidemic periods associated with different eras of human transportation (land, maritime and air travel) are shown. Overland trade networks and war campaigns are thought to have contributed to multiple epidemics in the Mediterranean in late classical antiquity (green), beginning with the Antonine plague, which reportedly claimed the life of the Roman emperor Lucius Verus 125 , 126 , 127 , 128 . Maritime transportation (red and grey) leading to European contact with the Americas and the subsequent Atlantic slave trade resulted in the importation of Plasmodium falciparum malaria and novel viral pathogens 129 . In modern times, air travel (purple) resulted in the importation of severe acute respiratory syndrome (SARS) coronavirus to 27 countries before transmission was halted 130 . b | In recent years, increases in air travel, trade and urbanization at global (left) and regional (right) scales have accelerated, indicating ever more frequent transport of people and goods between growing urban areas (source World Bank ). c | Log deaths from major epidemics in the twenty-first century (source World Health Organization ). d | Disability-adjusted life years lost from infectious diseases (source Our World in Data ). MERS, Middle East respiratory syndrome; NTD, neglected tropical disease.
Here, we review how recent anthropogenic climatic, demographic and technological changes have altered the landscape of infectious disease risk in the past two decades. In terms of climate change, we consider both the influence of recent warming and projected future changes. For demographic change, we include trends such as urbanization (Fig. 1b ), population growth, land-use change, migration, ageing and changing birth rates. For technological changes, we primarily consider advances that enable cheaper, faster global travel and trade (Fig. 1b ), as well as improved health care. We do not explicitly address economic change; however, economic changes, including economic development, are crucial drivers of these three factors: climate, demography and technology. We also do not explicitly discuss natural drivers of pathogen evolution or biological processes unless they interact with human-driven global change.
New infections chart a pathway beginning with emergence, followed by local-scale transmission, movement beyond borders and possible global-scale spread. Global changes may differentially affect the risk of emergence, the dynamics of disease within a local population and the global spread of diseases between populations. We provide an overview of each step, first considering features of recent global change that have altered the risks of spillover of viral, fungal, bacterial and apicomplexan (malaria) infections into human populations, then detailing how spread within human populations, driven by the seasonal dynamics of transmission, may be impacted by global change, of relevance to both emergent and established pathogens. Finally, we consider changes to the drivers of global spread, focusing in particular on travel, migration and animal and plant trade.
Pathogen emergence into human populations
Recent decades have seen repeated pathogen emergence from wild or domestic animal reservoirs into human populations, from HIV-1 and HIV-2, to the 1918 influenza virus, to Middle East respiratory syndrome coronavirus, to SARS-CoV-2 (refs 2 , 3 , 4 ). For a novel pathogen to become a threat to human populations, first, contact between humans and the animal reservoir must occur; the pathogen must either have or evolve (Box 1 ) the capacity for human-to-human transmission 5 ; and finally, this human-to-human transmission must enable expansion of the pathogen’s geographical range beyond the zone of spillover. Recent global changes have affected each of these steps.
Patterns of contact between human and wildlife reservoirs have increased as human populations move into previously unoccupied regions. Population growth and agricultural expansion, coupled with increasing wealth and larger property sizes, are driving factors for these interactions and the resulting habitat destruction. This may occur alongside behaviours that increase the potential for spillover, such as consumption of wild meat 6 , or intensifying contact between wild and domestic animal hosts. For example, Nipah virus has been identified in several bat populations, particularly flying foxes, but in 1999 caused a severe disease outbreak in Malaysia, primarily among pig farmers 7 . It is hypothesized that the spillover of Nipah virus from bats to pigs was driven by three factors related to global change: pig farms expanding into the bat habitat; intensification of pig farming, leading to a high density of hosts; and international trade, leading to the spread of the infection among other pig populations in Malaysia and Singapore 8 . Expanding agriculture and its intensification may create conditions that favour pathogen circulation within domestic animal (or plant) reservoirs via high-density farming practices 9 . Beyond creating opportunities for emergence of problematic livestock pathogens, this could also increase opportunities for evolution of novel variants of risk to humans in domestic animal reservoirs. This may occur alongside increasing risk to workers interacting with animal populations 10 as a result of work practices. Global increase in the demand for and resulting intensification of meat production will importantly drive these processes, and associated use of antibiotics in domestic animals has the potential to select for resistant strains of bacteria with potential to affect human health 11 .
The nature of human populations that are exposed to potential spillover is also changing. For example, the elimination of smallpox led to the cessation of smallpox vaccination, which may have enabled the expansion of monkeypox 12 . More generally, globally ageing populations may provide an immune landscape that is more at risk of spillover, as ageing immune landscapes are less capable of containing infectious agents 13 . The intersection between declining function of immunity at later ages 14 and globally ageing populations may increase the probability of pathogen emergence, but this remains conjectural and an important area for research. The changing global context may allow existing human pathogens to both evolve novel characteristics and expand in scope. Selection for drug resistance now occurs worldwide, and antibiotic resistance has and will evolve repeatedly 15 . As with antibiotic resistance, rapid global spread is commonplace for antimalarial resistance following evolution 16 .
Climate change may play a role in the risk from pathogen spillover. Changing environmental conditions can alter species range and density, leading to novel interactions between species, and increase the risk of zoonotic emergence 17 . A series of compounded environmental factors, including a long period of drought followed by extreme precipitation, is hypothesized to have driven an upsurge in rodent populations causing the emergence of pulmonary hantavirus in 1993 (ref. 18 ). Similarly, evidence suggests that populations of the black flying fox in Australia, a key reservoir of Hendra virus, have moved 100 km southward in the past 100 years owing to climatic changes. This shifting range likely caused Hendra virus to spill over into southern horse populations, and these horses subsequently infected humans 19 , 20 . Patterns of change are likely occurring in other bat populations globally but remain understudied — a clear cause for concern given the crucial role bat populations play as a reservoir host for several high-fatality pathogens 21 .
Rapid rates of urbanization in low-income and middle-income countries, and the increase in populations residing in crowded, low-quality dwellings, have created new opportunities for the emergence of infectious diseases (Fig. 2 ). Urbanization has promoted the emergence and spread of arboviral diseases such as dengue, Zika virus disease and chikungunya, which are transmitted by Aedes aegypti and Aedes albopictus mosquitoes that are well adapted to urban areas 22 , 23 , 24 . Population density appears correlated with the preference of Ae. aegypti for human odour, and hence the evolution of human-biting — the transmission pathway for arboviral disease 24 . However the role of urbanization in vector-borne disease spread is complex: the preference of the Anopheles spp. vector for rural environments may have led to a decline in the prevalence of malaria in urbanizing regions 25 . Nevertheless, dense and highly connected urban areas are potential hot spots for the rapid spread of diseases such as COVID-19 and SARS, and cities can serve as a catalyst for rapid local and global transmission.
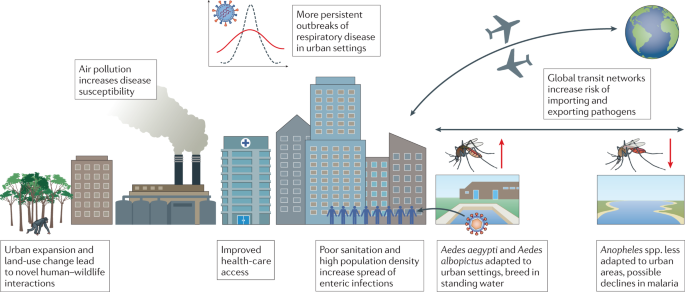
Interactions between urbanization and infectious disease are complex, with increased urbanization driving both positive and negative changes to global disease burden.
Box 1 Global change and evolution of hosts and pathogens
Mutations constantly arise in the genomes of all species, from viruses to elephants. Some genetic changes may have no observable effects on fitness (and thus will be selectively neutral), but can be used to track pathogen spread; for example, to trace the impacts of global connectivity on an outbreak 70 . Some genetic changes will affect disease phenotypes, potentially increasing the transmissibility, virulence or immune escape of a pathogen lineage 133 . The degree to which such mutations increase in frequency or spread geographically will depend on the degree to which they increase fitness, as well as pathogen population dynamics, which may be modulated by the global change context. Increases in the density and geographical distribution of susceptible hosts (whether they be people, crops or livestock) may provide greater opportunity for novel variants to emerge 9 simply by amplifying pathogen populations and thus circulating mutations. While understanding the nuance of cross-scale selection (that is, how the selective context of the individual host translates into the selective context at the scale of populations) remains a challenging frontier 134 , it is likely that ageing populations or the presence of immunosuppressive pathogens might further modulate selection pressures. Indeed, it has been suggested that the emergence of more transmissible or less immune-vulnerable variants of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was enabled in part by selection processes occurring during chronic infections in immunosuppressed individuals 135 . Greater global connectivity leads to more frequent exchange of this genetic material between populations of the same or different species, potentially leading to the erosion of evolved or engineered host resistance and increased rates of pathogen evolution 136 . Associated spillover followed by spillback can create scenarios that facilitate amplification and potentially selection of problematic pathogen variants 137 , an issue highlighted by recent documentation of human to mink to human transmission of SARS-CoV-2 (ref. 138 ). Likewise, increased rates of pathogen importation provide increased opportunities for pathogen populations to evolve the ability to utilize novel vectors (as has been observed in the Americas for malaria 129 ). Increased population connectivity can also enable pathogens and their vectors to shift to novel host species, from infected mosquitoes travelling on boats or in planes to agricultural pathogens being inadvertently relocated. Hosts that have not previously been exposed to such pathogens, and thus have no co-evolved defences, yet are phylogenetically and/or genetically similar to the original host are often most at risk 139 , 140 , a fact that makes homogenization of crops 141 or livestock a concern. Novel pathogen introductions can have large-scale population and ecosystem impacts, of which one famous example is the extirpation of the American chestnut tree by chestnut blight 142 . Changes in selection pressure resulting from changes in health-care strategies (for example, introduction of vaccination) may have the potential to select for different pathogen characteristics, and could potentially drive the evolution of virulence in pathogens 143 , 144 .
Local-scale disease dynamics
Emerging, re-emerging and endemic pathogens in human populations may exhibit distinct dynamic patterns of spread at the local scale. These patterns will be governed by demographic factors, including the effects of human behaviour on transmission (for example, school terms drive transmission of many childhood infections 26 and sex-specific travel patterns may result in higher burdens of chikungunya in women in Bangladesh 27 ) and immunity (which, for immunizing infections such as measles and rotavirus infection, is, in turn, shaped by replenishment of susceptible individuals via births 28 , 29 and depletion by vaccination where vaccines are available 30 ). Transmission may also be affected by climatic variables acting spatially or over the course of the year in line with seasonal fluctuations 31 , 32 . Recent global changes have affected each of these drivers of local-scale dynamics (Fig. 3 ).
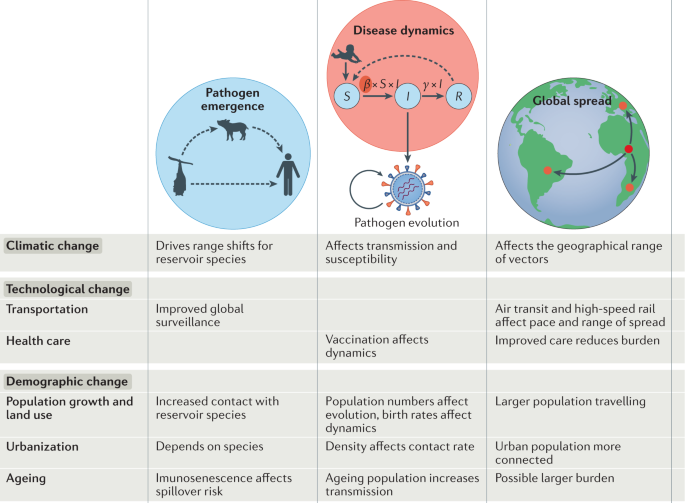
The table summarizes select recent global changes (rows) and their impacts on disease emergence, local-scale dynamics and global spread (columns). An example susceptible ( S ), infected ( I ), recovered ( R ) model is shown, where β represents the transmission rate and γ is the recovery rate.
As school attendance not only modulates transmission of childhood infections 26 but also shapes human mobility 33 , dramatic increases in rates of school attendance globally thus have the potential to substantially alter the dynamics of many infections. That this has yet to be documented is perhaps in part because this change has happened alongside expansion of access to vaccines that protect children against many of the relevant infections, as well as global declines in birth rates, which also facilitate control efforts by diminishing the size of the susceptible pool 34 . If the burden of disease is age specific, the intersection between immunity and shifting demography may be particularly marked: declining birth rates translate into a smaller pool of susceptible individuals and thus infected individuals, reducing the overall rate at which susceptible individuals become infected, and thus increasing the average age of infection or disease, as reported for dengue in Thailand 35 and rubella in Costa Rica 36 as these countries went through the demographic transition. Conversely, ageing populations may increase transmission; for example, longer shedding has been suggested with increasing age for SARS-CoV-2 (ref. 37 ).
Demographic changes to population size and density via urbanization may also affect dynamics. Influenza, for example, tends to exhibit more persistent outbreaks in more populous, denser urban regions 38 (Fig. 2 ). A similar pattern was reported in the early COVID-19 pandemic 39 . If demographic change has importantly altered the context of infectious diseases in recent years, arguably an even larger effect is caused by changes in the occurrence of immunomodulatory infections, which, in turn, may affect other infections. For example, the emergence of HIV has amplified the burden of tuberculosis 40 . Mass drug administration efforts have reduced helminth prevalence, which will have knock-on effects on the burden of other infections, such as malaria, which may be increased in individuals experiencing a heavy worm burden 41 ; both will also intersect with the efficacy of vaccination programmes 42 .
The climate plays a key role in driving the local-scale seasonal dynamics of many infectious diseases, which may thus be altered by global change in climatic conditions 43 , 44 . Considering these impacts requires recognizing that interactions with climate differ by pathogen type. For directly transmitted infections, the role of climate is revealed by marked latitudinal gradients in epidemic timing 32 , 45 . Several respiratory pathogens, including influenza virus, are more highly seasonal in temperate climates and exhibit greater year-round persistence in tropical locations 32 , 46 . Climate change is expected to lead to an expansion of these tropical patterns, with possible implications for pathogen evolution 43 , 47 . At the individual level, susceptibility to respiratory viral infections may be impacted by exposure to local air pollution, which is a concern for rapidly urbanizing locations, where urban air pollution may disproportionately affect low-income communities and communities of colour 48 , 49 . For example, non-Hispanic Black and Hispanic populations in the USA were found to have higher exposure to certain PM 2.5 components than non-Hispanic white populations 49 . At the same time, globally, a move to an urban location may bring benefits in terms of increased access to health care (Fig. 2 ).
For some bacterial and fungal diseases, climatic changes may affect the pathogen’s environmental reservoir. Incidence of coccidioidomycosis (valley fever), caused by inhalation of fungal spores of Coccidioides spp., is expected to increase with climate change as the region with optimal conditions for fungal spore production expands 50 . Climate change may also have played a role in the emergence of the drug-resistant fungal pathogen Candida auris . C. auris emerged in several continents at the same time and has been shown to have increased thermotolerance compared with other closely related fungal species, which perhaps evolved in response to global warming 51 , 52 . This increased thermotolerance may have enabled the pathogen to jump from its environmental habitat into an intermediary avian host, given the higher body temperatures of avian fauna, before infecting humans 52 .
Demographic change and technological changes may alter a host’s interaction with the environmental reservoir. Cholera, caused by the bacterial pathogen Vibrio cholerae , persists in the environment, particularly in aquatic settings. Changes to environmental conditions, including elevated sea temperatures, lead to increased reproduction of the pathogen and local epidemics 53 , with clear links to longer-term climate phenomena such as El Niño 54 . However, improved sanitation lowers the risk of exposure to V. cholerae and has led to a decline of the disease in many locations 53 .
For vector-transmitted diseases, biological traits of both the vector and the pathogen may be sensitive to climate. Many transmission-related life cycle traits of the mosquito (biting rate, adult lifespan, population size and distribution) and the pathogen (extrinsic incubation rate) are temperature sensitive, and oviposition patterns depend on water availability 55 . Consequently, the geographical range for dengue, malaria and other vector-borne diseases 56 , 57 , 58 is affected by the local climate, and there is substantial effort to understand how these ranges may change with climate change 59 , 60 , 61 . For certain vector-borne diseases such as Zika virus disease, climate change may lead to an expanded range 62 . However, for other diseases, such as malaria, climate change may shift the spatial range of the infection to higher latitudes 63 . As ever, the footprint of human interventions may loom larger than these changes in local conditions 25 .
At the local scale, one of the strongest footprints detectable on the dynamics of many endemic infections in recent years is declines in incidence associated with access to vaccinations 64 . However, the introduction of a vaccine does not imply immediate elimination. As vaccination coverage increases, measles outbreaks, for instance, follow a pathway towards elimination defined by declines in mean incidence but high variability in outbreak size 34 . Imperfect vaccine coverage may allow population susceptibility to increase such that substantial outbreaks can occur if the disease is reintroduced; for example, the 2018 measles outbreak in Madagascar, which led to more than 100,000 cases 65 . Improved surveillance of the landscape of population immunity, via serological surveys, could help determine gaps in vaccination coverage 66 .
Global spread
As local conditions alter demographically, or as a result of climate change potentially expanding the range of locations suitable to a particular pathogen or vector, increased global connectivity will enable pathogens to reach these new environments more rapidly (Figs 3 , 4 ). Here, we review the impact of global change on three forms of global connectivity — international travel, human migration and local-scale mobility, and the international trade of animals, animal products and plants — while considering the impact on infectious disease risk. Technological change over the past two decades has dramatically lowered the cost of international travel, while demographic change has led to heightened demand for inexpensive flights (Fig. 1b ). Demographic and climatic drivers have altered patterns of local mobility and regional migration, while rising demand and technological change have increased the trade of plants and animals. At the same time, an increasingly urban population is better connected than ever before to global travel networks (Fig. 4 ). These changes to global connectivity will present unique risk factors for infectious disease spread, enabling pathogens to travel further and faster than ever before.
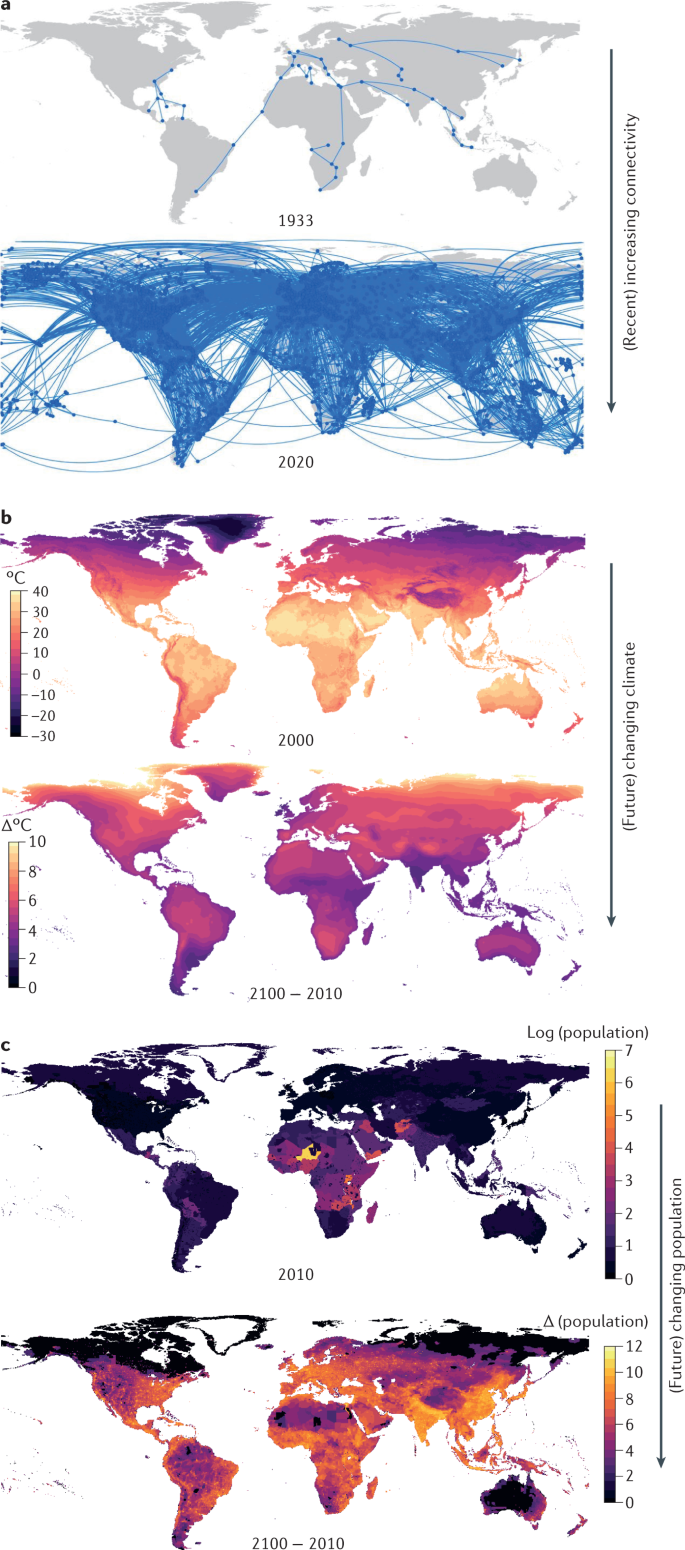
a | The global international air travel network expanded substantially from 1933 to 2020 (data from WorldPop and ref. 131 ). b | Average monthly maximum temperature in 1970–2000) and difference between 2070–2100 and 1970–2000 averages (data from WorldClim , Shared Socioeconomic Pathway 3 (SSP3)). c | Population projections under SSP3 in 2010 and relative population change projected until 2100 (source NASA Socioeconomic Data and Applications Center (ref. 132 )). Part a adapted with permission from ref. 131 , OUP.
International travel
The late twentieth century and the early twenty-first century have been marked by technological developments enabling ever swifter movement of people and pathogens over large distances — from trains to planes, and an expanding international airline network (Fig. 4 ). The total number of airline passengers doubled from just below two billion in 2000 to more than four billion in 2019 (Fig. 1b ). This rampant increase in global connectivity brings with it new risks from emerging pathogens (Box 2 ). However, many endemic pathogens also circulate via transit routes: seasonal influenza circulation in the USA can be predicted by flight patterns 67 , 68 , with evidence that flight bans following the events of 9/11 caused a delayed outbreak, and a prolonged influenza season within the USA as measured by a 60% increase in the time to transnational spread 68 . Similarly, rapid global air travel is expected to have played a key role in the global spread of SARS-CoV-2. Genetic analyses demonstrate multiple introductions of SARS-CoV-2, driven by air travel, in the Middle East 69 , northern California 70 and Brazil 71 .
International travel can lead to the global spread of vector-borne diseases via the introduction of new vectors into regions with suitable environmental conditions or the introduction of new pathogens into native and invasive vector populations. Historically, vectors have been introduced via trade routes: ships are thought to have been key to the global dispersal of Ae. aegypti and Ae. albopictus , which then became established in locations with appropriate environmental conditions 72 , 73 . Anopheles gambiae , the primary vector of malaria in Africa, was introduced into Brazil in the 1930s and became established in a region with a climate similar to that of its native Kenya 74 . Although malaria was already endemic in Brazil at the time, An. gambiae proved a much more effective vector, leading to a severe outbreak and a costly (but successful) eradication campaign 73 . There has been relatively little documented evidence of the introduction of new vectors via air travel. This is likely due to the low probability of vectors surviving the flight, and disembarking in a suitable region, in sufficient numbers to establish and drive an epidemic 75 . However, cases of ‘airport malaria’, that is, malaria transmitted within international airports, even outside endemic regions, are rare but becoming more common 76 .
A more feasible scenario is that air travel can bring an infected human host into contact with a native or invasive vector population that then establishes local transmission. Climate change has driven a shift in the range of several key vectors, which may make this introduction more likely. The range of the biting midge Culicoides imicola , a vector of bluetongue virus, which causes disease in ruminants, has expanded over the past few decades from sub-Saharan Africa and the Middle East into Europe, bringing a wave of bluetongue epidemics 77 . Following this expansion, bluetongue virus then spread outside the range of C. imicola into native populations of Culicoides spp. in more northerly regions of Europe. In terms of air travel, the 2015 Zika virus disease epidemic in the Americas may provide a recent example of a pathogen spreading into a susceptible vector population, likely facilitated by high connectivity 78 . Zika virus is thought to have been introduced to Brazil from French Polynesia and vectored by Aedes spp., although the volume of air travel during this period makes it almost impossible to conclusively determine the origin 78 . Similarly, it is hard to pinpoint the pathway via which West Nile virus was introduced into the USA in the 1990s; however, transport by either shipping (transporting vectors) or aircraft (transporting a human host) is likely 79 . After introduction, West Nile virus spread in the native Culex spp. mosquito population. More broadly, climate change complicates the picture in terms of possible future introductions. As the range of locations with environmental suitability for certain vector species changes, successful introductions of pathogens into these vector populations may become more likely 80 . At the same time, changes to population structure (for example, via urbanization) may alter the suitability of an environment for vector reproduction (Fig. 2 ).
Box 2 Will there be another pandemic like COVID-19?
COVID-19 has had an unprecedented impact on both human lives and our society, and we will likely be dealing with the consequences for decades to come. As we reckon with these consequences, one concern is that a suite of global changes has increased the risk from emerging pathogens, such that pandemics similar to COVID-19 could be a more frequent occurrence. However, there are biological features of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that have made the pathogen distinctly difficult to control, primarily the virus’s ability to spread asymptomatically and presymptomatically. Many pathogens do not exhibit these features, which may be a cause for cautious optimism going forward.
The expansion of regional and global air travel, along with the increasing development of high-speed railway networks, has resulted in a substantial degree of connectivity between human populations 73 . At the same time, land-use change and climate change may have increased the risk of pathogen emergence. In combination, these drivers imply an era where pathogens are more likely to emerge, and more likely to spread globally on emergence. However, while the last century bore witness to several pandemics (Fig. 1 ), SARS-CoV-2 is unrivalled in its rapid, global reach. A key question is why SARS-CoV-2 was so successful at spreading globally and whether this was due to recent increases in global connectivity as opposed to epidemiological and biological characteristics of the virus itself 145 .
A clear distinction between SARS-CoV-2 and other recently emerged pathogens (for example, SARS-CoV and Ebola virus) is that an individual infected with SARS-CoV-2 may become infectious before developing symptoms 146 . This presents a unique challenge from a disease control perspective. A standard approach for limiting the onward spread of a new outbreak is to isolate infected individuals when they show symptoms. Case isolation proved successful in mitigating earlier SARS 147 and Ebola virus disease 148 outbreaks. However, symptoms for SARS-CoV-2 infection likely occur after an individual is already infectious 146 . This possible presymptomatic spread limits the efficacy of case isolation interventions as by the time the infected individual is isolated, the person may have already spread the pathogen to others 149 . In the figure, we plot the time to infectiousness (latent period) against the time to symptom onset (incubation period) for four pathogens that have caused severe outbreaks in recent decades. When the latent period equals the incubation period (dashed line in the figure), symptoms occur at a similar time to infectiousness (for example, influenza). The shaded region to the right of this line in the figure indicates possible presymptomatic spread, which may be uniquely difficult to control.
The 2–3-day delay between infectiousness and symptom onset provides ample time for long-distance spread of the disease, given current transport networks (see the figure). Control policies, such as testing before travel, provide a more effective option in this context, yet developing and distributing a test takes time, during which time the disease may spread rapidly. The good news is that this presymptomatic spread appears somewhat unique to SARS-CoV-2, at least compared with other acute infections such as influenza, SARS and Ebola virus disease (Fig. 4 ). In comparison, asymptomatic spread explains some of the difficulty in controlling acquired immunodeficiency syndrome before antiretroviral measures were available.
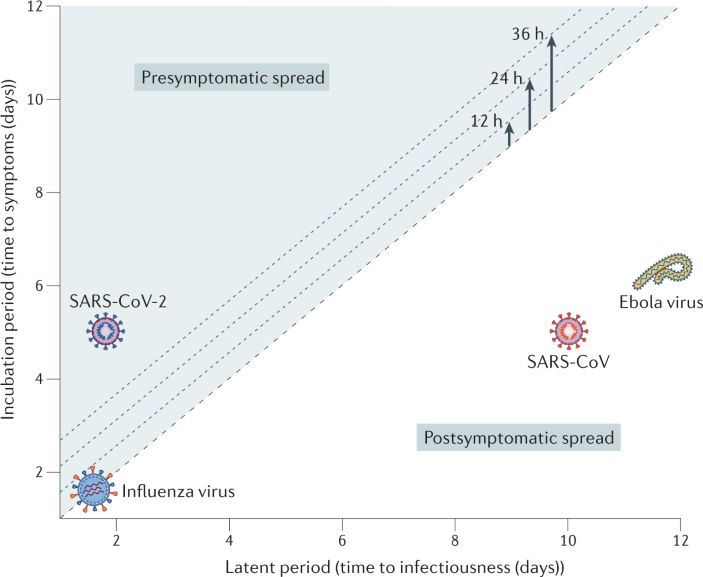
Migration and local mobility
Human migration is an intrinsic component of population dynamics driven by socio-economic, political and environmental factors, and one that has undergone considerable upheaval in the modern era. It is estimated that globally the number of international migrants, those who intentionally relocate to a country other than their birth country, is almost 272 million, representing 3.5% of the world’s population. By contrast, temporary migration, often considered ‘seasonal migration’, is driven largely by economic patterns, including agricultural seasons that require short periods of intense labour. The rate of migration continues to increase owing to both social, economic, political and environmental drivers in origin countries and economic opportunities, physical safety and security in destination countries. Projections for migration are unclear, with the UN projecting stable rates after 2050 (ref. 81 ). However, climate change will likely provide an escalating push factor, with sea level rise and extreme weather events leading to forced migration from exposed regions 82 .
Given the movement of people between countries, there remain risks of introduction of infectious diseases, including those common and uncommon in the country of migration 83 . It is possible for a infectious disease common in the source country, such as latent tuberculosis, malaria, viral hepatitis and infection with intestinal parasites, to be imported via this mechanism 84 , 85 , 86 . For example, in many destination countries, a large proportion of cases of tuberculosis are observed in the foreign-born population. However, the ultimate impact of these introduction events will depend largely on the population-level susceptibility and environmental suitability for sustained transmission in the destination country. More importantly, migrant groups often have more limited access to health care, treatment and resources, particularly those displaced, who are often provided with limited options to safely seek care and treatment 87 . Minimizing the impact of these possible disease threats depends on providing appropriate health care to these high-risk groups that takes into account the multifaceted social, political and economic components 88 .
Within-country population mobility can also play a key role in disease spread; however, it is typically difficult to track these movements. Aggregated mobile phone data are a valuable tool for tracing patterns of local mobility and predicting future outbreaks 89 . In recent work, mobility data have been shown to be predictive of inequities in COVID-19 burden in the USA 90 . Similarly, population mobility was found to predict the spread of the 2011 dengue epidemic in Pakistan 91 , while local travel following the Eid holidays was found to predict the spread of the chikungunya outbreak in 2017 in Bangladesh 92 . As the trend of urbanization continues, mobility to and from dense urban centres (that is, megacities) will likely play a future role in local spread of infections 92 . Better tracking of within-country population mobility, using novel data streams, may present an opportunity for forecasting future outbreaks 93 .
Intensification of animal and plant trade
International trade has expanded rapidly in the modern era and has been matched by a global proliferation of infectious diseases affecting not only humans but also animals and plants 94 , 95 . Trade drives this pattern by facilitating the translocation of hosts and pathogens across the geographical and ecological boundaries that constrain their spread. The economic and environmental threats posed by trade-driven infectious diseases of plants and animals are increasingly being recognized, and calls for more stringent containment measures have intensified in recent years 96 , 97 .
Plant trade
Deliberate transport of plant products has existed since the emergence of trade. Increases in the speed of transport during modern times have allowed more live plant tissue, and as a result more viable pathogen propagules , to be transported over long distances. Combined with the intensification of trade at the global scale, this pattern has driven a rise in long-distance transmission and disease emergence 98 , 99 . Trade drives the emergence of novel plant diseases by creating novel interactions between hosts and pathogens 100 . One pathway through which this can occur is the introduction of novel pathogens to native plants. For example, Xylella fastidiosa , a generalist bacterium vectored by xylem-feeding insects, was introduced into Europe in 2013 from the USA, likely as a result of trade. In Italy, X. fastidiosa is causing an ongoing epidemic of ‘olive quick decline syndrome’, resulting in severe losses of an economically and culturally important crop 101 , 102 . Trade can also drive the emergence of plant disease by introducing novel hosts to native pathogens. Eucalyptus rust, a disease caused by the fungal pathogen Austropuccinia psidii , emerged when the pathogen transferred from its native South American hosts in the myrtle family (Myrtaceae) to non-native Eucalyptus trees (which also belong to the myrtle family) being grown on plantations 103 . The disease now threatens to ‘spill back’ into naive endemic Eucalyptus populations in Australia.
Animal and animal-product trade
Animal trade has contributed to multiple outbreaks and emergence events globally, which have had major consequences for the agricultural sector as a whole and pose substantial risk for animal and public health. Large numbers of livestock are traded annually between countries and may facilitate the spread of pathogens. Rift Valley fever, for example, is a zoonotic vector-borne viral disease causing abortion and high neonatal mortality in domestic ruminants. The disease is widespread on the African continent and has recently been detected in Saudi Arabia and Yemen. Live cattle movement between East Africa and the Arabian peninsula or from the Union of Comoros to Madagascar is thought to have contributed to the introduction of Rift Valley fever virus and caused outbreaks in these locations in 2000 (Arabian Peninsula) and 2008 (Madagascar) 104 , 105 .
Additionally, the trade of animal-derived products such as meat may enable the movement of pathogens over large distances and between continents. For instance, African swine fever is a highly contagious viral disease affecting several members of the family Suidae, including domestic pigs and wild boars. Infection by African swine fever virus may result in up to 100% morbidity and mortality in affected pig herds and substantial economic losses for producers. In 2007, the accidental introduction of African swine fever virus to Georgia led to the first outbreak of African swine fever in Europe since the early 1990s 106 . The virus, which used to occur primarily in sub-Saharan Africa, was allegedly introduced to the Caucasian peninsula through meat products contaminated with viruses closely related to the ones found in Madagascar, Mozambique or Zambia 107 . Despite efforts to contain the virus, the disease has spread to more than 20 countries in Europe and Asia 108 , 109 .
Similarly, in recent decades there has been an expansion in infections of Vibrio parahaemolyticus — a bacterial pathogen found in shellfish and the leading cause of seafood-related illness globally. The pathogen is endemic to regions of the US Pacific Northwest but has recently spread to other parts of the USA, Europe and South America 110 , 111 . The concerning increase in V. parahaemolyticus infection is expected to have several drivers connected to global change. Declines in sea ice have increased ship traffic through the Bering Strait, with cargo ships possibly transporting V. parahaemolyticus in ballast water. At the same time, increasing sea temperatures may have increased the global environmental suitability for V. parahaemolyticus in the marine environment 110 . Finally, dispersal of the pathogen may have occurred via increasing global trade in shellfish, with evidence suggesting possible dispersal via Manila clams introduced into Spain from Canada 111 . This combination of possible drivers speaks to the complexity of understanding infectious disease risk in an era of global change, and the necessity of exploring concurrent changes.
Transboundary spread of diseases through legal and illegal trade of live animals may also have important consequences for biodiversity on a global scale. For example, the amphibian trade contributed to the expansion of novel strains of the fungal pathogen genus Batrachochytrium into naive hosts, devastating wild amphibian populations globally 112 . Conversely, infectious diseases also hamper trade, resulting in indirect economic losses in affected populations. Foot and mouth disease virus is a major reason for trade restrictions on livestock. While endemic in certain countries in Asia and Africa, foot and mouth disease virus causes outbreaks in naive populations, resulting in large economic losses 113 . While trade is a major driver of pathogen spread, food animal production has transformed in recent history into large-scale intensified systems with high-density, genetically homogenous populations, ideal for pathogen emergence and maintenance 114 . Critically, animal production systems often serve as the interface between wild and human populations, and multiple viral spillover events have occurred at this nexus. Nipah virus spilled over from fruit bats to the domestic pig population multiple times before subsequently infecting humans 115 . Pandemic variants of human influenza A virus are often the result of reassortment between human and avian viruses, with both domestic poultry and wild birds posited to play a role 116 , 117 , 118 . A non-viral example is the spillover of antimicrobial-resistant pathogens from livestock into humans: intensive antibiotic use in industrialized and smallholder livestock production systems to promote growth and prevent infections has been linked to the emergence of antibiotic resistance in humans 119 . Tackling emergence and disease spread in animal systems will require rethinking both food animal production and global trade of animals.
A new era of infectious disease
In recent decades, declines in mortality and morbidity, particularly childhood mortality, have been one of the great triumphs of public health. Greater access to care, such as therapeutics (including antibiotics), improved sanitation and the development of vaccines 120 have been core drivers of this progress. Even as medical advances in the twenty-first century have spurred advances in population health, inequalities in access to these advances remain widespread between and within countries 121 . Reducing inequities in access to health care and improving surveillance and monitoring for infectious diseases in low-income and middle-income countries, and in underserved populations within countries, should be a priority in tackling pathogen emergence and spread.
While life expectancy continues to increase, and life years lost to infectious diseases decline, the new threat of infectious disease will likely come from emerging and re-emerging infections. Climate change, rapid urbanization and changing land-use patterns will increase the risk of disease emergence in the coming decades. Climate change, in particular, may alter the range of global pathogens, allowing infections, particularly vector-borne infections, to expand into new locations. A continued uptick in global travel, trade and mobility will transport pathogens rapidly, following emergence. However, there are counterpoints to this trend: the rapid growth of connectivity observed in the early twenty-first century may stabilize, and structural changes wrought during the COVID-19 pandemic may persist 122 . Increased investment in outbreak response, such as the recent formation of the WHO Hub for Pandemic and Epidemic Intelligence, could help mitigate the threat from future emerging infections. In addition, efforts to develop universal vaccines (that is, vaccines that engender immunity against all strains of influenza viruses or coronaviruses, for example) could provide a monumental leap forward in tackling present and future infections 123 .
A changing world requires changing science to evaluate future risks from infectious disease. Future work needs to explicitly address concurrent changes: how shifting patterns of demographic, climatic and technological factors may collectively affect the risk of pathogen emergence, alterations to dynamics and global spread. More forward-looking research, to contend with possible future outcomes, is required in addition to the retroactive analyses that typically dominate the literature. Increasing attention needs to be paid to pathogens currently circulating in both wild and domestic animal populations, especially in cases where agriculture is expanding into native species’ habitats and, conversely, invasive species are moving into populous regions due to climate change. As the battle against certain long-term endemic infections is won, institutional structures built to address these old enemies can be co-opted and adapted for emerging threats. At the same time, new technologies, including advances in data collection and surveillance, need to be harnessed (Box 3 ). There is much recent innovation around surveillance, from reinterpreting information available from classic tools such as PCR 124 to leveraging multiplex serology approaches to identify anomalies that might suggest pathogen emergence, and there is increasing interest in integrating multiple surveillance platforms (from genomic to case data) to better understand pathogen spread. Finally, future research needs to align with a global view of disease risk. In an increasingly connected world, the risk from infectious disease is globally shared. The COVID-19 pandemic, including the rapid global circulation of evolved strains, highlights the need for a collaborative, worldwide framework for infectious disease research and control.
Box 3 Big data for disease
Recent technological advances in collecting, sharing and processing large datasets, from satellite images to genomes, represent a new opportunity to answer critical questions in global health. However, challenges remain, including the uneven geographical distribution of available data as well as biases in representative sampling. We highlight three areas of future growth.
Serological surveys
Serological surveys detect the presence of antibodies in blood — recent advances in testing now enable the detection of exposure to multiple pathogens with use of a small sample of blood 150 . Serological surveys have attracted attention during the COVID-19 pandemic as a means to track population exposure given under-reporting, although test performance characteristics differ widely between epidemiological contexts as well as the choice of assay used 151 . Historically, serological surveys have been financially and logistically expensive to run, but declining costs are leading to increased availability of serological data.
Genomic surveillance systems
Genomic surveillance systems are able to characterize and track the emergence of novel variants (for example, during the COVID-19 pandemic). Undoubtedly these data have enabled the rapid development of diagnostics and vaccines and, when combined with epidemiological information, are able to provide a more detailed picture of ongoing transmission dynamics. Efforts to develop national and international genomic surveillance networks are varied but with clear success stories 152 , 153 even in low-resources settings 154 . However, resource limitations, including sequencing platforms, bioinformatic pipelines and the regular collection of samples for processing, continue to limit the global expansion of sequencing.
Artificial intelligence and machine learning
These techniques are frequently proposed as tools for answering key public health questions, yet specific use cases remain elusive 155 . Using these tools to predict viral emergence, for example, may prove difficult due to microbiological complexities and the cost of data collection 156 , yet could prove valuable for targeting sampling efforts 157 . In terms of uncovering population-level drivers of disease transmission, statistical approaches, including machine learning, can be used to leverage novel, and high-volume, data streams. However, more classical, mechanistic models may provide a more robust framework for projecting future outcomes for the disease system under demographic, technological and climatic change. Future work should aim to improve the integration of machine learning approaches within the traditional mechanistic modelling frameworks to rapidly and accurately assess prospective challenges.
Roeder, P., Mariner, J. & Kock, R. Rinderpest: the veterinary perspective on eradication. Philos. Trans. R. Soc. Lond. B Biol. Sci. 368 , 20120139 (2013).
PubMed PubMed Central Google Scholar
Karesh, W. B. et al. Ecology of zoonoses: natural and unnatural histories. Lancet 380 , 1936–1945 (2012).
Plowright, R. K. et al. Pathways to zoonotic spillover. Nat. Rev. Microbiol. 15 , 502–510 (2017).
CAS PubMed PubMed Central Google Scholar
Zhou, P. & Shi, Z.-L. SARS-CoV-2 spillover events. Science 371 , 120–122 (2021).
CAS PubMed Google Scholar
Lloyd-Smith, J. O. et al. Epidemic dynamics at the human-animal interface. Science 326 , 1362–1367 (2009).
Brashares, J. S. et al. Bushmeat hunting, wildlife declines, and fish supply in West Africa. Science 306 , 1180–1183 (2004).
Parashar, U. D. et al. Case-control study of risk factors for human infection with a new zoonotic paramyxovirus, Nipah Virus, during a 1998–1999 outbreak of severe encephalitis in Malaysia. J. Infect. Dis. 181 , 1755–1759 (2000).
Field, H. et al. The natural history of Hendra and Nipah viruses. Microbes Infect. 3 , 307–314 (2001).
Pitzer, V. E. et al. High turnover drives prolonged persistence of influenza in managed pig herds. J. R. Soc. Interface 13 , 20160138 (2016).
Jones, B. A. et al. Zoonosis emergence linked to agricultural intensification and environmental change. Proc. Natl Acad. Sci. USA 110 , 8399–8404 (2013).
van Boeckel, T. et al. Global trends in antimicrobial resistance in animals in low-and middle-income countries. Int. J. Infect. Dis. 101 , 19 (2020).
Google Scholar
Rimoin, A. W. et al. Major increase in human monkeypox incidence 30 years after smallpox vaccination campaigns cease in the Democratic Republic of Congo. Proc. Natl Acad. Sci. USA 107 , 16262–16267 (2010).
Aw, D., Silva, A. B. & Palmer, D. B. Immunosenescence: emerging challenges for an ageing population. Immunology 120 , 435–446 (2007).
Nikolich-Žugich, J. The twilight of immunity: emerging concepts in aging of the immune system. Nat. Immunol. 19 , 10–19 (2018).
PubMed Google Scholar
Laxminarayan, R. et al. Antibiotic resistance-the need for global solutions. Lancet Infect. Dis. 13 , 1057–1098 (2013).
Talisuna, A. O., Bloland, P. & D’Alessandro, U. History, dynamics, and public health importance of malaria parasite resistance. Clin. Microbiol. Rev. 17 , 235–254 (2004).
Carlson, C. J. et al. Climate change will drive novel cross-species viral transmission. Preprint at bioRxiv https://doi.org/10.1101/2020.01.24.918755 (2020).
Article PubMed PubMed Central Google Scholar
Patz, J. A., Epstein, P. R., Burke, T. A. & Balbus, J. M. Global climate change and emerging infectious diseases. JAMA 275 , 217–223 (1996).
Martin, G. et al. Climate change could increase the geographic extent of Hendra virus spillover risk. Ecohealth 15 , 509–525 (2018).
Yuen, K. Y. et al. Hendra virus: epidemiology dynamics in relation to climate change, diagnostic tests and control measures. One Health 12 , 100207 (2021). Martin et al. (2018) and Yuen et al. (2021) detail the link between climate change and recent Hendra virus spillover .
Brook, C. E. et al. Accelerated viral dynamics in bat cell lines, with implications for zoonotic emergence. eLife 9 , e48401 (2020).
Gubler, D. J. Dengue, urbanization and globalization: the unholy trinity of the 21st century. Trop. Med. Health 39 , S3–S11 (2011).
Li, Y. et al. Urbanization increases Aedes albopictus larval habitats and accelerates mosquito development and survivorship. PLoS Negl. Trop. Dis. 8 , e3301 (2014).
Rose, N. H. et al. Climate and Urbanization drive mosquito preference for humans. Curr. Biol. 30 , 3570–3579.e6 (2020).
Tatem, A. J., Gething, P. W., Smith, D. L. & Hay, S. I. Urbanization and the global malaria recession. Malar. J. 12 , 133 (2013).
Mahmud, A. S., Metcalf, C. J. E. & Grenfell, B. T. Comparative dynamics, seasonality in transmission, and predictability of childhood infections in Mexico. Epidemiol. Infect. 145 , 607–625 (2017).
Salje, H. et al. How social structures, space, and behaviors shape the spread of infectious diseases using chikungunya as a case study. Proc. Natl Acad. Sci. USA 113 , 13420–13425 (2016). This article shows how interactions between the characteristics of an individual and that individual’s environment contribute to disease dynamics .
Pitzer, V. E. et al. Demographic variability, vaccination, and the spatiotemporal dynamics of rotavirus epidemics. Science 325 , 290–294 (2009).
Earn, D. J., Rohani, P., Bolker, B. M. & Grenfell, B. T. A simple model for complex dynamical transitions in epidemics. Science 287 , 667–670 (2000).
Ferrari, M. J., Grenfell, B. T. & Strebel, P. M. Think globally, act locally: the role of local demographics and vaccination coverage in the dynamic response of measles infection to control. Philos. Trans. R. Soc. Lond. B Biol. Sci. 368 , 20120141 (2013).
Thompson, C. N. et al. The impact of environmental and climatic variation on the spatiotemporal trends of hospitalized pediatric diarrhea in Ho Chi Minh City, Vietnam. Health Place. 35 , 147–154 (2015).
Baker, R. E. et al. Epidemic dynamics of respiratory syncytial virus in current and future climates. Nat. Commun. 10 , 5512 (2019).
Wesolowski, A. et al. Multinational patterns of seasonal asymmetry in human movement influence infectious disease dynamics. Nat. Commun. 8 , 2069 (2017).
Graham, M. et al. Measles and the canonical path to elimination. Science 364 , 584–587 (2019).
Cummings, D. A. T. et al. The impact of the demographic transition on dengue in Thailand: insights from a statistical analysis and mathematical modeling. PLoS Med. 6 , e1000139 (2009).
Metcalf, C. J. E. et al. Structured models of infectious disease: inference with discrete data. Theor. Popul. Biol. 82 , 275–282 (2012).
Cevik, M. et al. SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: a systematic review and meta-analysis. Lancet Microbe 2 , e13–e22 (2021).
Dalziel, B. D. et al. Urbanization and humidity shape the intensity of influenza epidemics in U.S. cities. Science 362 , 75–79 (2018). This study describes distinct patterns of influenza outbreaks in urban locations .
Rader, B. et al. Crowding and the shape of COVID-19 epidemics. Nat. Med. 26 , 1829–1834 (2020).
Kwan, C. K. & Ernst, J. D. HIV and tuberculosis: a deadly human syndemic. Clin. Microbiol. Rev. 24 , 351–376 (2011).
Nkuo-Akenji, T. K., Chi, P. C., Cho, J. F., Ndamukong, K. K. J. & Sumbele, I. Malaria and helminth co-infection in children living in a malaria endemic setting of mount Cameroon and predictors of anemia. J. Parasitol. 92 , 1191–1195 (2006).
Hartmann, W. et al. Helminth infections suppress the efficacy of vaccination against seasonal influenza. Cell Rep. 29 , 2243–2256.e4 (2019).
Mahmud, A. S., Martinez, P. P., He, J. & Baker, R. E. The impact of climate change on vaccine-preventable diseases: insights from current research and new directions. Curr. Env. Health Rep. 7 , 384–391 (2020).
Baker, R. E., Yang, W., Vecchi, G. A., Metcalf, C. J. E. & Grenfell, B. T. Assessing the influence of climate on wintertime SARS-CoV-2 outbreaks. Nat. Commun. 12 , 846 (2021).
Tamerius, J. D. et al. Environmental predictors of seasonal influenza epidemics across temperate and tropical climates. PLoS Pathog. 9 , e1003194 (2013).
Viboud, C., Alonso, W. J. & Simonsen, L. Influenza in tropical regions. PLoS Med. 3 , e89 (2006).
Baker, R. E. et al. Implications of climatic and demographic change for seasonal influenza dynamics and evolution. Preprint at medRxiv https://doi.org/10.1101/2021.02.11.21251601 (2021).
Ciencewicki, J. & Jaspers, I. Air pollution and respiratory viral infection. Inhal. Toxicol. 19 , 1135–1146 (2007).
Bell, M. L. & Ebisu, K. Environmental inequality in exposures to airborne particulate matter components in the United States. Environ. Health Perspect. 120 , 1699–1704 (2012).
Gorris, M. E., Treseder, K. K., Zender, C. S. & Randerson, J. T. Expansion of coccidioidomycosis endemic regions in the United States in response to climate change. Geohealth 3 , 308–327 (2019). This study is one of the first to describe the link between climate change and valley fever .
Du, H. et al. Candida auris: epidemiology, biology, antifungal resistance, and virulence. PLoS Pathog. 16 , e1008921 (2020).
Casadevall, A., Kontoyiannis, D. P. & Robert, V. On the emergence of Candida auris: climate change, azoles, swamps, and birds. mBio 10 , e01397 (2019).
Colwell, R. R. Global climate and infectious disease: the cholera paradigm. Science 274 , 2025–2031 (1996).
Koelle, K., Rodó, X., Pascual, M., Yunus, M. & Mostafa, G. Refractory periods and climate forcing in cholera dynamics. Nature 436 , 696–700 (2005). This study highlights the importance of the interplay between intrinsic (temporary immunity) and extrinsic (climatic variability) factors in determining disease dynamics .
Mordecai, E. A. et al. Detecting the impact of temperature on transmission of Zika, dengue, and chikungunya using mechanistic models. PLoS Negl. Trop. Dis. 11 , e0005568 (2017).
Rocklöv, J. & Dubrow, R. Author correction: climate change: an enduring challenge for vector-borne disease prevention and control. Nat. Immunol. 21 , 695 (2020).
Brady, O. J. et al. Global temperature constraints on Aedes aegypti and Ae. albopictus persistence and competence for dengue virus transmission. Parasit. Vectors 7 , 338 (2014).
Kraemer, M. U. G. et al. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. eLife 4 , e08347 (2015).
Hales, S., de Wet, N., Maindonald, J. & Woodward, A. Potential effect of population and climate changes on global distribution of dengue fever: an empirical model. Lancet 360 , 830–834 (2002).
Wagner, C. E. et al. Climatological, virological and sociological drivers of current and projected dengue fever outbreak dynamics in Sri Lanka. J. R. Soc. Interface 17 , 20200075 (2020).
Couper, L. I., MacDonald, A. J. & Mordecai, E. A. Impact of prior and projected climate change on US Lyme disease incidence. Glob. Chang. Biol. 27 , 738–754 (2021).
Ryan, S. J. et al. Warming temperatures could expose more than 1.3 billion new people to Zika virus risk by 2050. Glob. Chang. Biol. 27 , 84–93 (2021).
Ryan, S. J., Lippi, C. A. & Zermoglio, F. Shifting transmission risk for malaria in Africa with climate change: a framework for planning and intervention. Malar. J. 19 , 170 (2020).
Li, X. et al. Estimating the health impact of vaccination against ten pathogens in 98 low-income and middle-income countries from 2000 to 2030: a modelling study. Lancet 397 , 398–408 (2021).
Mensah, K. et al. Seasonal gaps in measles vaccination coverage in Madagascar. Vaccine 37 , 2511–2519 (2019).
Metcalf, C. J. E. et al. Use of serological surveys to generate key insights into the changing global landscape of infectious disease. Lancet 388 , 728–730 (2016).
Crépey, P. & Barthélemy, M. Detecting robust patterns in the spread of epidemics: a case study of influenza in the United States and France. Am. J. Epidemiol. 166 , 1244–1251 (2007).
Brownstein, J. S., Wolfe, C. J. & Mandl, K. D. Empirical evidence for the effect of airline travel on inter-regional influenza spread in the United States. PLoS Med. 3 , e401 (2006).
Tayoun, A. A. et al. Multiple early introductions of SARS-CoV-2 into a global travel hub in the Middle East. Sci. Rep. 10 , 17720 (2020).
Deng, X. et al. Genomic surveillance reveals multiple introductions of SARS-CoV-2 into northern California. Science 369 , 582–587 (2020).
Candido, D. S. et al. Evolution and epidemic spread of SARS-CoV-2 in Brazil. Science 369 , 1255–1260 (2020).
Lounibos, L. P. Invasions by insect vectors of human disease. Annu. Rev. Entomol. 47 , 233–266 (2002).
Tatem, A. J., Hay, S. I. & Rogers, D. J. Global traffic and disease vector dispersal. Proc. Natl Acad. Sci. USA 103 , 6242–6247 (2006).
Killeen, G. F., Fillinger, U., Kiche, I., Gouagna, L. C. & Knols, B. G. J. Eradication of Anopheles gambiae from Brazil: lessons for malaria control in Africa? Lancet Infect. Dis. 2 , 618–627 (2002).
Tatem, A. J. et al. Air travel and vector-borne disease movement. Parasitology 139 , 1816–1830 (2012).
Huang, Z. & Tatem, A. J. Global malaria connectivity through air travel. Malar. J. 12 , 269 (2013).
Purse, B. V., Rogers, D. J., Mellor, P. S., Baylis, M. & Mertens, P. P. C. Bluetongue virus and climate change. in Bluetongue (eds Mellor, P. S., Baylis, M. & Mertens, P. P. C.) 343–364 (Elsevier, 2009). This study describes the role of climate change in the geographical expansion of bluetongue epidemics .
Massad, E. et al. On the origin and timing of Zika virus introduction in Brazil. Epidemiol. Infect. 145 , 2303–2312 (2017).
Kilpatrick, A. M. Globalization, land use, and the invasion of West Nile virus. Science 334 , 323–327 (2011).
Mordecai, E. A., Caldwell, J. M. & Grossman, M. K. Thermal biology of mosquito-borne disease. Ecol. Lett. 22 , 1690–1708 (2019).
United Nations. World Population Prospects 2019 (2019).
Wrathall, D. J. et al. Meeting the looming policy challenge of sea-level change and human migration. Nat. Clim. Chang. 9 , 898–901 (2019).
Gushulak, B. D. & MacPherson, D. W. Globalization of infectious diseases: the impact of migration. Clin. Infect. Dis. 38 , 1742–1748 (2004).
Soto, S. M. Human migration and infectious diseases. Clin. Microbiol. Infect. 15 (Suppl. 1), 26–28 (2009).
Monge-Maillo, B. et al. Imported infectious diseases in mobile populations, Spain. Emerg. Infect. Dis. 15 , 1745–1752 (2009).
Castelli, F. & Sulis, G. Migration and infectious diseases. Clin. Microbiol. Infect. 23 , 283–289 (2017).
Bhatia, A. et al. The Rohingya in Cox’s Bazar: when the stateless seek refuge. Health Hum. Rights 20 , 105–122 (2018).
Chin, T., Buckee, C. O. & Mahmud, A. S. Quantifying the success of measles vaccination campaigns in the Rohingya refugee camps. Epidemics 30 , 100385 (2020).
Wesolowski, A., Buckee, C. O., Engø-Monsen, K. & Metcalf, C. J. E. Connecting mobility to infectious diseases: the promise and limits of mobile phone data. J. Infect. Dis. 214 , S414–S420 (2016).
Chang, S. et al. Mobility network models of COVID-19 explain inequities and inform reopening. Nature 589 , 82–87 (2021). This study uses high-resolution mobility data to explain inequities in COVID-19 burden .
Wesolowski, A. et al. Impact of human mobility on the emergence of dengue epidemics in Pakistan. Proc. Natl Acad. Sci. USA 112 , 11887–11892 (2015).
Mahmud, A. S. et al. Megacities as drivers of national outbreaks: The 2017 chikungunya outbreak in Dhaka, Bangladesh. PLoS Negl. Trop. Dis. 15 , e0009106 (2021).
Buckee, C. O. et al. Aggregated mobility data could help fight COVID-19. Science 368 , 145–146 (2020).
Perrings, C. Options for managing the infectious animal and plant disease risks of international trade. Food Security 8 , 27–35 (2016).
Smith, K. F. et al. Ecology. Reducing the risks of the wildlife trade. Science 324 , 594–595 (2009).
Perrings, C., Levin, S. & Daszak, P. The economics of infectious disease, trade and pandemic risk. Ecohealth 15 , 241–243 (2018).
Pavlin, B. I., Schloegel, L. M. & Daszak, P. Risk of importing zoonotic diseases through wildlife trade, United States. Emerg. Infect. Dis. J. 15 , 1721 (2009).
Santini, A., Liebhold, A., Migliorini, D. & Woodward, S. Tracing the role of human civilization in the globalization of plant pathogens. ISME J. 12 , 647–652 (2018).
Levine, J. M. & D’Antonio, C. M. Forecasting biological invasions with increasing international trade. Conserv. Biol. 17 , 322–326 (2003). This article gives a quantitative forecast of the impact of international trade on the introduction of plant pathogens .
Parker, I. M. & Gilbert, G. S. The evolutionary ecology of novel plant-pathogen interactions. Annu. Rev. Ecol. Evol. Syst. 35 , 675–700 (2004).
Landa, B. B. et al. Emergence of a plant pathogen in Europe associated with multiple intercontinental introductions. Appl. Environ. Microbiol. https://doi.org/10.1128/AEM.01521-19 (2020).
Frisullo, S., Camele, I., Agosteo, G. E., Boscia, D. & Martelli, G. P. Brief historical account of olive leaf scorch (‘brusca’) in the Salento Peninsula of Italy and state-of-the-art of the olive quick decline syndrome. J. Plant. Pathol. 96 , 441–449 (2014).
Coutinho, T. A., Wingfield, M. J., Alfenas, A. C. & Crous, P. W. Eucalyptus rust: a disease with the potential for serious international implications. Plant. Dis. 82 , 819–825 (1998).
Shoemaker, T. et al. Genetic analysis of viruses associated with emergence of Rift Valley fever in Saudi Arabia and Yemen, 2000-01. Emerg. Infect. Dis. 8 , 1415–1420 (2002).
Lancelot, R. et al. Drivers of Rift Valley fever epidemics in Madagascar. Proc. Natl Acad. Sci. USA 114 , 938–943 (2017).
Costard, S., Mur, L., Lubroth, J., Sanchez-Vizcaino, J. M. & Pfeiffer, D. U. Epidemiology of African swine fever virus. Virus Res. 173 , 191–197 (2013).
Rowlands, R. J. et al. African swine fever virus isolate, Georgia, 2007. Emerg. Infect. Dis. 14 , 1870–1874 (2008).
Mighell, E. & Ward, M. P. African swine fever spread across Asia, 2018-2019. Transbound. Emerg. Dis. https://doi.org/10.1111/tbed.14039 (2021).
Article PubMed Google Scholar
European Food Safety Authority (EFSA). et al. Epidemiological analysis of African swine fever in the European Union (September 2019 to August 2020). EFSA J. 19 , e06572 (2021).
Abanto, M., Gavilan, R. G., Baker-Austin, C., Gonzalez-Escalona, N. & Martinez-Urtaza, J. Global expansion of Pacific Northwest Vibrio parahaemolyticus sequence type 36. Emerg. Infect. Dis. 26 , 323–326 (2020).
Martinez-Urtaza, J. et al. Genomic variation and evolution of ´Vibrio parahaemolyticus ST36 over the course of a transcontinental epidemic expansion. mBio https://doi.org/10.1128/mBio.01425-17 (2017). Abanto et al. (2020) and Martinez-Urtaza et al. (2017) discuss how global trade and climate change have led to the expansion of V. parahaemolyticus .
Fisher, M. C. & Garner, T. W. J. Chytrid fungi and global amphibian declines. Nat. Rev. Microbiol. 18 , 332–343 (2020).
Knight-Jones, T. J. D. & Rushton, J. The economic impacts of foot and mouth disease - what are they, how big are they and where do they occur? Prev. Vet. Med. 112 , 161–173 (2013).
Robinson, T. P. et al. Antibiotic resistance: mitigation opportunities in livestock sector development. Animal 11 , 1–3 (2017).
Pulliam, J. R. C. et al. Agricultural intensification, priming for persistence and the emergence of Nipah virus: a lethal bat-borne zoonosis. J. R. Soc. Interface 9 , 89–101 (2012).
Rambaut, A. et al. The genomic and epidemiological dynamics of human influenza A virus. Nature 453 , 615–619 (2008).
Olsen, B. et al. Global patterns of influenza a virus in wild birds. Science 312 , 384–388 (2006).
Li, Y. et al. Continued evolution of H5N1 influenza viruses in wild birds, domestic poultry, and humans in China from 2004 to 2009. J. Virol. 84 , 8389–8397 (2010).
Food and Agriculture Organization of the United Nations. Drivers, Dynamics and Epidemiology of Antimicrobial Resistance in Animal Production . (Food and Agriculture Organization, 2018).
Metcalf, C. J. E. et al. Transport networks and inequities in vaccination: remoteness shapes measles vaccine coverage and prospects for elimination across Africa. Epidemiol. Infect. 143 , 1457–1466 (2015).
Hotez, P. J. Globalists versus nationalists: bridging the divide through blue marble health. PLoS Negl. Trop. Dis. 13 , e0007156 (2019).
Antràs, P. De-globalisation? Global value chains in the post-COVID-19 age. National Bureau of Economic Research https://www.nber.org/papers/w28115 (2020).
Burton, D. R. & Topol, E. J. Variant-proof vaccines — invest now for the next pandemic. Nature https://doi.org/10.1038/d41586-021-00340-4 (2021).
Hay, J. A. et al. Estimating epidemiologic dynamics from cross-sectional viral load distributions. Science https://doi.org/10.1126/science.abh0635 (2021).
Wagner, D. M. et al. Yersinia pestis and the Plague of Justinian 541–543 AD: a genomic analysis. Lancet Infect. Dis. 14 , 319–326 (2014).
Alfani, G. & Murphy, T. E. Plague and lethal epidemics in the pre-industrial world. J. Econ. Hist. 77 , 314–343 (2017).
Harper, K. Pandemics and passages to late antiquity: rethinking the plague of c. 249–270 described by Cyprian. J. Rom. Archaeol. 28 , 223–260 (2015).
Duncan-Jones, R. P. The impact of the Antonine plague. J. Rom. Archaeol. 9 , 108–136 (1996).
Molina-Cruz, A., Zilversmit, M. M., Neafsey, D. E., Hartl, D. L. & Barillas-Mury, C. Mosquito vectors and the globalization of Plasmodium falciparum malaria. Annu. Rev. Genet. 50 , 447–465 (2016).
Guan, Y. et al. Molecular epidemiology of the novel coronavirus that causes severe acute respiratory syndrome. Lancet 363 , 99–104 (2004).
Tatem, A. J. Mapping population and pathogen movements. Int. Health 6 , 5–11 (2014).
Gao, J. Global 1-km downscaled population base year and projection grids based on the shared socioeconomic pathways, revision 01. NASA Socioeconomic Data and Applications Center (SEDAC) https://doi.org/10.7927/q7z9-9r69 (2020).
Article Google Scholar
Miller, I. F. & Metcalf, C. J. E. Evolving resistance to pathogens. Science 363 , 1277–1278 (2019).
Park, M., Loverdo, C., Schreiber, S. J. & Lloyd-Smith, J. O. Multiple scales of selection influence the evolutionary emergence of novel pathogens. Philos. Trans. R. Soc. Lond. B Biol. Sci. 368 , 20120333 (2013).
Kemp, S. A. et al. SARS-CoV-2 evolution during treatment of chronic infection. Nature 592 , 277–282 (2021).
Zhan, J., Thrall, P. H., Papaïx, J., Xie, L. & Burdon, J. J. Playing on a pathogen’s weakness: using evolution to guide sustainable plant disease control strategies. Annu. Rev. Phytopathol. 53 , 19–43 (2015).
Olival, K. J. et al. Possibility for reverse zoonotic transmission of SARS-CoV-2 to free-ranging wildlife: a case study of bats. PLoS Pathog. 16 , e1008758 (2020).
Oude Munnink, B. B. et al. Transmission of SARS-CoV-2 on mink farms between humans and mink and back to humans. Science 371 , 172–177 (2021).
Shaw, L. P. et al. The phylogenetic range of bacterial and viral pathogens of vertebrates. Mol. Ecol. 29 , 3361–3379 (2020).
Gilbert, G. S. & Webb, C. O. Phylogenetic signal in plant pathogen–host range. Proc. Natl Acad. Sci. USA 104 , 4979–4983 (2007).
Gibson, A. K. & Nguyen, A. E. Does genetic diversity protect host populations from parasites? A meta-analysis across natural and agricultural systems. Evol. Lett. 5 , 16–32 (2021).
Rigling, D. & Prospero, S. Cryphonectria parasitica, the causal agent of chestnut blight: invasion history, population biology and disease control. Mol. Plant. Pathol. 19 , 7–20 (2018).
Gandon, S., Mackinnon, M. J., Nee, S. & Read, A. F. Imperfect vaccines and the evolution of pathogen virulence. Nature 414 , 751–756 (2001).
Miller, I. F. & Metcalf, C. J. Vaccine-driven virulence evolution: consequences of unbalanced reductions in mortality and transmission and implications for pertussis vaccines. J. R. Soc. Interface 16 , 20190642 (2019).
Fraser, C., Riley, S., Anderson, R. M. & Ferguson, N. M. Factors that make an infectious disease outbreak controllable. Proc. Natl Acad. Sci. USA 101 , 6146–6151 (2004).
Bar-On, Y. M., Flamholz, A., Phillips, R. & Milo, R. Science forum: SARS-CoV-2 (COVID-19) by the numbers. eLife 9 , e57309 (2020).
Hollingsworth, T. D., Ferguson, N. M. & Anderson, R. M. Will travel restrictions control the international spread of pandemic influenza? Nat. Med. 12 , 497–499 (2006).
Dhillon, R. S., Srikrishna, D. & Sachs, J. Controlling Ebola: next steps. Lancet 384 , 1409–1411 (2014).
Hellewell, J. et al. Feasibility of controlling COVID-19 outbreaks by isolation of cases and contacts. Lancet Glob. Health 8 , e488–e496 (2020).
Xu, G. J. et al. Viral immunology. Comprehensive serological profiling of human populations using a synthetic human virome. Science 348 , aaa0698 (2015). This study presents a highly multiplexed, peptide-level serological assay to characterize prior exposure to all known viruses .
Takahashi, S., Greenhouse, B. & Rodríguez-Barraquer, I. Are SARS-CoV-2 seroprevalence estimates biased? J. Infect. Dis. https://doi.org/10.1093/infdis/jiaa523 (2020).
The Lancet. Genomic sequencing in pandemics. Lancet 397 , 445 (2021).
COVID-19 Genomics UK (COG-UK) [email protected]. An integrated national scale SARS-CoV-2 genomic surveillance network. Lancet Microbe 1 , e99–e100 (2020).
Inzaule, S. C., Tessema, S. K., Kebede, Y. & Ouma, A. E. O. Genomic-informed pathogen surveillance in Africa: opportunities and challenges. Lancet Infect. Dis. https://doi.org/10.1016/S1473-3099(20)30939-7 (2021).
Jordan, M. I. & Mitchell, T. M. Machine learning: trends, perspectives, and prospects. Science 349 , 255–260 (2015).
Holmes, E. C. What can we predict about viral evolution and emergence? Curr. Opin. Virol. 3 , 180–184 (2013).
Carlson, C. J. From PREDICT to prevention, one pandemic later. Lancet Microbe 1 , e6–e7 (2020).
Download references
Acknowledgements
R.E.B. is supported by the Cooperative Institute for Modelling Earth Systems. R.E.B., C.J.E.M. and F.R. are supported by the High Meadows Environmental Institute at Princeton University. A.W. is supported by the US National Institutes of Health through the National Library of Medicine (DP2LM013102) and the National Institute of Allergy and Infectious Diseases (1R01A1160780-01) and a Career Award at the Scientific Interface from the Burroughs Wellcome Fund. Research in the L.-F.W. group is supported by grants from the Singapore National Research Foundation (NRF2012NRF-CRP001-056 and NRF2016NRF-NSFC002-013), the National Medical Research Council of Singapore (MOH-OFIRG19MAY-0011, COVID19RF-003 and NMRC/BNIG/2040/2015) and the Ministry of Education, Singapore (MOE2019-T2-2-130). A.J.T. is supported by the Bill & Melinda Gates Foundation (INV-024911). S.T. is supported by the Schmidt Science Fellows programme, in partnership with the Rhodes Trust.
Author information
Authors and affiliations.
Department of Ecology and Evolutionary Biology, Princeton University, Princeton, NJ, USA
Rachel E. Baker, Ian F. Miller, Malavika Rajeev, Fidisoa Rasambainarivo, Benjamin L. Rice & C. Jessica E. Metcalf
Princeton High Meadows Environmental Institute, Princeton University, Princeton, NJ, USA
Rachel E. Baker & Fidisoa Rasambainarivo
Department of Demography, University of California, Berkeley, Berkeley, CA, USA
Ayesha S. Mahmud
Rocky Mountain Biological Laboratory, Crested Butte, CO, USA
Ian F. Miller
Mahaliana Labs SARL, Antananarivo, Madagascar
Fidisoa Rasambainarivo
Madagascar Health and Environmental Research (MAHERY), Maroantsetra, Madagascar
Benjamin L. Rice
EPPIcenter Program, Division of HIV, ID, and Global Medicine, Department of Medicine, University of California, San Francisco, San Francisco, CA, USA
Saki Takahashi
WorldPop, School of Geography and Environmental Science, University of Southampton, Southampton, UK
Andrew J. Tatem
Department of Bioengineering, McGill University, Montreal, Quebec, Canada
Caroline E. Wagner
Programme in Emerging Infectious Diseases, Duke-NUS Medical School, Singapore, Singapore
Lin-Fa Wang
Duke Global Health Institute, Duke University, Durham, NC, USA
Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Johns Hopkins University, Baltimore, MD, USA
Amy Wesolowski
Princeton School of Public and International Affairs, Princeton University, Princeton, NJ, USA
C. Jessica E. Metcalf
You can also search for this author in PubMed Google Scholar
Contributions
R.E.B., B.L.R. and A.J.T. researched data for the article. All authors contributed substantially to discussion of the content, wrote the article and edited and reviewed the manuscript before submission.
Corresponding authors
Correspondence to Rachel E. Baker or C. Jessica E. Metcalf .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Peer review information.
Nature Reviews Microbiology thanks Matthew Baylis and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
NASA Socioeconomic Data and Applications Center: https://sedac.ciesin.columbia.edu/
Our World in Data: https://ourworldindata.org/
World Bank: https://data.worldbank.org/
WorldClim: https://www.worldclim.org/
World Health Organization: https://www.who.int/
WorldPop: https://www.worldpop.org/
A correlated series of climate events associated with the warm phase of the El Niño Southern Oscillation cycle.
Pathogen units responsible for infection, such as a fungal spore or viral particle.
The mixing of genetic material of different pathogens within an infected cell.
The measurement of antibody responses to multiple pathogens simultaneously.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Baker, R.E., Mahmud, A.S., Miller, I.F. et al. Infectious disease in an era of global change. Nat Rev Microbiol 20 , 193–205 (2022). https://doi.org/10.1038/s41579-021-00639-z
Download citation
Accepted : 17 September 2021
Published : 13 October 2021
Issue Date : April 2022
DOI : https://doi.org/10.1038/s41579-021-00639-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
The effect of bovine dairy products and their components on the incidence and natural history of infection: a systematic literature review.
- Meghan Mitchell
- Sarah S. Cohen
Nutrition Journal (2024)
Intranasal delivery of PEA-producing Lactobacillus paracasei F19 alleviates SARS-CoV-2 spike protein-induced lung injury in mice
- Alessandro Del Re
- Silvia Basili Franzin
- Giuseppe Esposito
Translational Medicine Communications (2024)
A methodology for estimating SARS-CoV-2 importation risk by air travel into Canada between July and November 2021
- Rachael M. Milwid
- Vanessa Gabriele-Rivet
- Erin E. Rees
BMC Public Health (2024)
Pine wilt disease: what do we know from proteomics?
- Joana M. S. Cardoso
- Bruno Manadas
- Luís Fonseca
BMC Plant Biology (2024)
Incidence and mortality of community-acquired and nosocomial infections in Japan: a nationwide medical claims database study
- Nozomi Takahashi
- Taro Imaeda
- Taka‑aki Nakada
BMC Infectious Diseases (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Microbiology newsletter — what matters in microbiology research, free to your inbox weekly.
Dengue fever, once confined to the tropics, now threatens the U.S.
Meg Norris was traveling in Argentina in April when the first signs of dengue fever hit her. The weather in Salta, just south of the Bolivian border, was warm, but Norris, a 33-year-old from Boulder, Colorado, zipped a fleece sweatshirt around her body to stop herself from shivering.
“I thought it was sun poisoning,” she said.
She woke that night in a sweat and spent the hours alternately burning up then freezing. In the morning, her eyes were sore and her lymph nodes were swollen. For the following week, there was nothing to do but sleep, stay hydrated and wait for the body aches that give the illness the moniker “break-bone fever” to pass.

Latin America is experiencing its worst dengue fever outbreak on record. Case numbers in the first 4 ½ months of 2024 are already 238% higher than they were by this time last year, which itself ended with a record 4.1 million cases, according to the Pan American Health Organization . Cases are more than 400% higher than the five-year average.
An unusually wet and warm summer season brought by the El Niño weather pattern has created ideal conditions for the mosquitoes that spread dengue to hatch en masse and carry higher amounts of the virus.
Experts warn this could be a preview of what dengue fever will look like in the future. Climate change is creating unusually balmy conditions, which are already expanding the range of mosquito-borne diseases.
“That’s concerning for places where dengue hasn’t occurred before in recent history: North America and Europe,” said Dr. Albert Ko, a professor of epidemiology of microbial diseases at the Yale School of Public Health.
Dengue is a viral fever caused by four different viruses and spread through mosquito bites. It’s common in many tropical regions across the globe, but has begun to appear in more temperate climates. The mosquitoes that carry dengue fever, Aedes aegypti are now regularly found in the southern parts of the U.S., but recently, the insects have been found as far north as the Bay Area and Washington, D.C. One 2019 study predicted an additional 2 billion people will be at risk for dengue fever by 2080.
“We are definitely worried,” Ko said.
Why are dengue cases rising around the world?
Dengue outbreaks have historically occurred in the Americas every three to four years, said Dr. Gabriela Paz-Bailey, dengue branch chief in the division of vector-borne diseases at the Centers for Disease Control and Prevention. “But now we are seeing them every year,” she said.
Part of the reason for that is tied to climate change.
A warming climate expands the mosquitoes’ habitat and allows them to breed all year long, rather than only in the warmer months. The hotter temperatures also cause the viruses to replicate faster, meaning mosquitoes end up carrying many more viral copies, increasing the likelihood that a person will become infected if bitten.
“We are also seeing dengue cause outbreaks at times when they usually don’t occur,” Ko said.
South America’s dengue cases weren’t just unusually high this year, but they also came unusually early in the season. Similarly, Puerto Rico, a place where dengue outbreaks can occur in the summer and fall, declared a public health emergency in late March after the U.S. territory was overtaken by dengue fever cases and more than 400 people were hospitalized .
In recent years, the epidemic has spread to parts of southern Brazil and northern Argentina, where dengue hasn’t previously been a big problem, Ko said.
“That gives us a snapshot of what we may see here in North America in the coming decades,” Ko said.
How would dengue get a foothold in the U.S.?
The fact that Aedes aegypti mosquitoes are found in places outside their normal range doesn’t mean the mosquitoes are carrying dengue viruses, but those first insects are a warning of what may be to come, Ko added
Locally transmitted dengue fever infections — meaning the infected person didn’t get sick abroad — are still rare in the continental U.S., but have recently been seen for the first time in some states. Last October, California health officials reported the state’s first case of locally transmitted dengue in Pasadena. Local transmission has also occurred in Arizona, Florida and the southern coast of Texas. Last summer brought record-breaking heat waves to Europe, where cases of local dengue transmission were seen in France, Italy and Spain.
“I think this means dengue will become more common,” said Paz-Bailey, adding that the main concern is still the significant increase in cases where the virus is already endemic.
This summer, she does not expect to see significant dengue outbreaks on the U.S. mainland, but she said there is likely to be some people who travel to regions that have higher-than-usual cases and bring the virus back home.
“Travel-associated cases do result in small chains of outbreaks,” Paz-Bailey said.
Humans are reservoirs for dengue, so in order to have widespread transmission, enough people must be infected for the mosquitoes to reliably bite someone with the virus so that they can spread it to another person.
“That’s why we’re seeing an outbreak of dengue in Puerto Rico right now,” said Michael von Fricken, director of the One Health Center of Excellence at the University of Florida in Gainesville. “They’ve reached this tipping point where there are enough infected humans that they’re subsequently infecting other mosquitoes that are continuing to transmit disease.”
Florida has logged 176 dengue cases so far this year, the vast majority in people who were infected in other countries, most frequently Brazil or Cuba. The Florida Health Department has recorded only seven cases of locally transmitted dengue transmission in the state so far this year. In all of 2023, the department documented 173 locally transmitted cases, most of them in Miami-Dade County.
What are the symptoms of dengue fever?
Dengue fever is caused by four viruses, so a person can be infected four times in their lifetime. Only about 1 in 4 people are symptomatic the first time they’re infected, according to the CDC.
Ko said a person’s initial symptoms are usually a fever and headaches. Fatigue, nausea, vomiting, a rash that looks like measles, as well as the extremely painful body aches.
Most people recover in a week or two, but about 1 in 20 people develop severe dengue, which can be fatal. The more times a person is infected with dengue, the higher risk they are for complications.
“After you’ve had your first exposure, your risk of having dengue hemorrhagic fever or severe symptoms increases exponentially,” Von Fricken said. Dengue also becomes deadlier with each infection.
While the U.S. does have a dengue vaccine, it’s approved only for children ages 9 to 16 who live in places where dengue is endemic, including Puerto Rico, American Samoa or the U.S. Virgin Islands.
What’s more, children can get the vaccine only if they’ve previously had a dengue infection. That’s because if a person were to get vaccinated and then get their first dengue infection, they still run the risk of getting very sick, just as someone gets sicker from their second infection. Since most Americans have not had dengue, “that vaccine is not very useful” for most, Ko said.
There’s no specific drug to treat dengue. Instead, doctors just aim to treat the symptoms and keep the patient comfortable until the virus runs its course. That means resting and drinking a lot of fluids. Ko said people should try to take acetaminophen (Tylenol) for pain and fever if they can, since nonsteroidal anti-inflammatory drugs (NSAIDs), which include ibuprofen and aspirin, can make bleeding worse if someone develops hemorrhagic dengue, in which their blood vessels are damaged and become leaky.
Paz-Bailey said it’s important for people traveling to places with dengue to stay in places with air conditioning when possible, use insect-repellant and wear long sleeves and pants to avoid mosquito bites.
Bed nets can be helpful, but the mosquitoes that carry dengue typically bite during the day, so they may be less helpful than they are at preventing other mosquito-borne diseases like malaria, Ko said.
At home, people can make their yards less appealing to mosquitos by reducing the amount of standing water, especially after a bout of rain.
“It’s difficult to control the mosquito population, so we need to hit it with all we have and layer our strategies,” Paz-Bailey said. “No single strategy will be good enough.”
Kaitlin Sullivan is a contributor for NBCNews.com who has worked with NBC News Investigations. She reports on health, science and the environment and is a graduate of the Craig Newmark Graduate School of Journalism at City University of New York.
Anne Thompson is NBC News’ chief environmental affairs correspondent.
Patrick Martin is a producer in the NBC News Health & Medical Unit.
MORE SECTIONS
- Dear Deidre
MORE FROM THE SUN
- Newsletters

- News Travel
Emergency landing as flight diverted to Dublin Airport after medical issue & infectious disease unit evacuate plane
- Macarten Burke
- Published : 18:56, 3 Jun 2024
- Updated : 9:52, 4 Jun 2024
A BUSY jet heading from Brussels to New York was forced to make an unscheduled stop at Dublin Airport due to two separate incidents on board.
The United Airlines plane was due to land at Newark Airport in New York but was forced to land in the capital due to a suspected medical issue and a disruptive passenger this afternoon.

Gardai , National Ambulance Service and HSE personnel were notified and met the aircraft on arrival.
The HSE infectious disease unit boarded the flight at Dublin Airport.
Appropriate protocols were enforced by Dublin Airport Operations and Airport Police to ensure the safety of passengers on the flight as they dealt with the two incidents.
The details of the suspected medical issue and disruptive passenger, which forced the flight to make an unscheduled landing, have not yet been revealed.
READ MORE ON TRAVEL NEWS

Urgent warning for Dublin Airport passengers flying out during busy weekend

Save money at Dublin Airport with six ‘game-changer’ packing hacks
The unscheduled landing has no impact on the operations at the airport, and the passengers arriving in the capital should be at normal times with no delays.
The Dublin Airport Authority released a statement on X (formerly Twitter ) about the unscheduled landing.
The statement reads: "Flight UA998 from Brussels to New York's Newark Airport diverted to @DublinAirport this afternoon due to reports of two separate incidents - a disruptive passenger and a suspected medical issue - on board.
"Appropriate protocols were put in place by Dublin Airport Operations and Airport Police for the arrival of the aircraft and Gardai, National Ambulance Service and HSE personnel met the aircraft on arrival.
Most read in News Travel

Ryanair forced to cancel nearly 100 flights tomorrow due to 'inexplicable' strikes

Major ‘reached limit’ tourist ban fears for ANOTHER popular holiday hotspot

Aer Lingus announce major sale to top sunshine spot with €5 pints & 27C heat

Brits arrive on holiday with 'no hotel bookings' after tour operator goes bust
"There is no impact on operations at Dublin Airport and passengers travelling this evening should plan to arrive at the airport as normal."
In a statement, the HSE said they "cannot comment on individual cases" to protect the privacy and confidentiality of the people involved.
They added: "Departments of Public Health respond to notified cases of suspected infectious diseases, undertake a risk assessment of the situation and control measures are put in place, if required, to protect the public health.
"Today, HSE National Ambulance Service and HSE Public Health undertook appropriate investigations at Dublin Airport following protocols in partnership with Dublin Airport, An Garda Síochána and Airport Police.
"Public Health and National Ambulance Service managed this incident according to national public health guidelines."
Separately, Dublin Airport has seen its busiest day over the Bank Holiday Weekend , as over 100,000 passengers passed through.
Taking to X, formerly Twitter , the airport said: "A busy Sunday at Dublin Airport with more than 115,000 passengers passing through. A warm welcome awaits for all those flying today."
In the picture a huge pride flag can be seen welcoming arrivals , with the words: "DAA celebrating Pride".
Busiest weekend at Dublin Airport
It was expected that over 450,000 people would pass through the airport this June Bank Holiday.
The four day period between last Friday and today, Monday saw 240,000 passengers departing from Dublin Airport with 210,000 passengers arriving.
The busiest day was yesterday, Sunday June 2 when 117,000 passengers were expected to travel through the airport.
The car parks at Dublin Airport were sold out for the entire weekend.
Those without an existing booking were advised to travel to the airport via an alternate mode such as a bus or taxi.
Spaces are still available in the short-term car parks for people dropping off and picking up passengers.
- Planes flights and airlines
- Dublin Airport
- New York City
- United States
You are using an outdated browser. Upgrade your browser today or install Google Chrome Frame to better experience this site.
What is COVID-19?
Who can get covid-19, can i travel if i recently had covid-19, what can travelers do to prevent covid-19, more information.
CDC Respiratory Virus Guidance has been updated. The content of this page will be updated soon.
Coronavirus disease 2019 (COVID-19) is a respiratory illness caused by the virus SARS-CoV-2. The virus spreads mainly from person to person through respiratory droplets and small particles produced when an infected person coughs, sneezes, or talks. The virus spreads easily in crowded or poorly ventilated indoor settings.
People with COVID-19 have reported a wide range of symptoms – ranging from no or mild symptoms to severe illness. Symptoms may appear 2–14 days after exposure to the virus. Possible symptoms include fever, chills, cough, shortness of breath, fatigue, muscle aches, headache, new loss of taste and smell, sore throat, runny nose, nausea, vomiting, or diarrhea.
Anyone can get COVID-19. However, some people are more likely than others to get very sick if they get COVID-19. These include people who are older, are immunocompromised , or have certain disabilities , or have underlying health conditions . Vaccination, past infection, and timely access to testing and treatment can help protect you from getting very sick from COVID-19.
Yes, you can travel once you have ended isolation . Check CDC guidance for additional precautions, including testing and wearing a mask around others. If you recently had COVID-19 and are recommended to wear a mask, do not travel on public transportation such as airplanes, buses, and trains if you are unable to wear a mask whenever around others.
Get up to date with your COVID-19 vaccines before you travel and take steps to protect yourself and others . Consider wearing a mask in crowded or poorly ventilated indoor areas, including on public transportation and in transportation hubs. Take additional precautions if you were recently exposed to a person with COVID-19. Don’t travel while sick.
If you have a weakened immune system or are at increased risk for severe disease talk to a healthcare professional before you decide to travel. If you travel, take multiple prevention steps to provide additional layers of protection from COVID-19, even if you are up to date with your COVID-19 vaccines. These include improving ventilation and spending more time outdoors, avoiding sick people, getting tested for COVID-19 if you develop symptoms, staying home if you have or think you have COVID-19, and seeking treatment if you have COVID-19.
Consider getting travel insurance in case you need medical care abroad .
Consider getting a COVID-19 test if you:
- Develop COVID-19 symptoms before, during, or after travel.
- Will be traveling to visit someone who is at higher risk of getting very sick from COVID-19.
- Were in a situation with a greater risk of exposure during travel (e.g., in an indoor, crowded space like an airport terminal while not wearing a mask).
If you traveled and feel sick, particularly if you have a fever, talk to a healthcare professional, and tell them about your recent travel.
- Masking During Travel
File Formats Help:
- Adobe PDF file
- Microsoft PowerPoint file
- Microsoft Word file
- Microsoft Excel file
- Audio/Video file
- Apple Quicktime file
- RealPlayer file
- Zip Archive file
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.

Rising measles cases across Europe prompt travel and vaccination warnings for UK families
P arents planning summer getaways are being urgently advised to ensure their children are vaccinated as measles cases have seen a dramatic rise in recent months, affecting 17 popular holiday destinations across Europe.
The European Centre for Disease Prevention (ECDP) reported that a staggering 1,671 cases of the potentially fatal disease were recorded in April alone, with Romania, Poland and Belgium being some of the hardest hit areas, reporting 957, 84 and 76 cases respectively.
France, Austria, The Netherlands, Ireland, Spain and Portugal have all witnessed an upsurge in the illness. Additionally, a smaller number of cases have been detected in Sweden, Lithuania, Cyprus, Denmark, Greece, Croatia, Hungary and Estonia.
- READ MORE: Drug dealer had addicts queueing in alleyways waiting to buy crack cocaine
- READ MORE: Daughter rives mum's hair for turning off Wi-Fi as attack captured on dog-cam
These alarming figures follow the report of 10,887 measles cases across Europe over the past year, resulting in seven fatalities from the infection. A significant majority of these infections, 87%, occurred in individuals who had not been vaccinated.
Health experts are now strongly encouraging parents to consider administering the MMR vaccine to their children in an effort to reduce the risk of infection and potential severe complications should they contract the disease. Earlier this month, the UK Health Security Agency (UKHSA) issued a warning to parents to remain vigilant following a substantial increase in UK cases at the beginning of the year.
This year, England has been hit by a significant measles outbreak, with almost 900 cases reported in 2024 so far, dwarfing the 368 cases recorded in 2023. The country has experienced more instances of the disease in the first four months of this year than in the entirety of the previous year, reports Wales Online .
"Most of the cases have been in London, although there have been cases picked up across the country and some are linked to travel abroad," according to the UK Health Security Agency (UKHSA).
Alarmingly, vaccination rates for the potentially fatal illness have plummeted to a 12-year low, with just 84% of children receiving both doses of the MMR vaccine (which protects against Measles, Mumps, and Rubella) by the age of five. To effectively prevent outbreaks and safeguard those who cannot be vaccinated, coverage needs to exceed 90%.
Dr Helen Bedford, Professor of Children's Health at UCL, has expressed concern over the inadequate vaccine uptake, warning that measles cases will continue to escalate if the trend does not reverse. She remarked: "This is the biggest outbreak of measles we've had since 2012. We've had more cases in the first four months of this year than in the whole of last year."
"The main reason for that is vaccine uptake figures for all vaccines, not just MMR figures have been going down very slightly year-on-year for the last ten years. Our uptake for the MMR vaccines overall for England is 89.3% for the first dose and 84.5% for the second dose."
"But the big, important thing about measles is that it is incredibly infectious. It is the most infectious respiratory disease around, and because of that, we need to have very very high levels of vaccine uptake. We need at least 95% for both doses."
What are the symptoms of measles?
Measles typically starts off presenting cold-like symptoms such as a heightened temperature, blocked or runny nose, sneezing, coughing and red watery eyes, which are then generally followed by a skin rash after a few days.
In some instances, individuals may also develop small spots inside their mouth. These smaller white spots tend to appear on the cheeks' interior and at the back of the lips. They typically last just a few days.
The skin rash normally makes an appearance a few days following the initial cold-like symptoms. It usually starts on the face and behind the ears before gradually spreading across the rest of the body.
The measles rash can sometimes be raised and merge together to form irregularly-shaped patches, but it's not normally itchy. On pale skin, the rash tends to appear brownish or reddish. It might be harder to spot against darker complexions.
How can I prevent measles?
If you're looking to prevent contracting measles, the best method is through getting the MMR vaccine. This combines protection from not only measles, but also mumps and rubella. In the UK, all children are offered two doses of this vaccine which should ensure they receive lifelong coverage from all three diseases.
However, some individuals, including pregnant women, those with a weakened immune system, or those who have had a severe allergy to any components of the jab, such as neomycin or gelatine, are unable to receive the vaccination.

- Search Menu
- Sign in through your institution
- Advance articles
- Editor's Choice
- Supplement Archive
- Cover Archive
- IDSA Guidelines
- IDSA Journals
- The Journal of Infectious Diseases
- Open Forum Infectious Diseases
- Photo Quizzes
- State-of-the-Art Reviews
- Voices of ID
- Author Guidelines
- Open Access
- Why Publish
- Advertising and Corporate Services
- Advertising
- Journals Career Network
- Reprints and ePrints
- Sponsored Supplements
- Branded Books
- About Clinical Infectious Diseases
- About the Infectious Diseases Society of America
- About the HIV Medicine Association
- IDSA COI Policy
- Editorial Board
- Self-Archiving Policy
- For Reviewers
- For Press Offices
- Journals on Oxford Academic
- Books on Oxford Academic
Article Contents
The use of long-term antibiotics for suppression of bacterial infections.
- Article contents
- Figures & tables
- Supplementary Data
Molly Horne, Ian Woolley, Jillian S Y Lau, The use of long-term antibiotics for suppression of bacterial infections, Clinical Infectious Diseases , 2024;, ciae302, https://doi.org/10.1093/cid/ciae302
- Permissions Icon Permissions
Suppressive antibiotic therapy is prescribed when a patient has an infection that is presumed to be incurable by a defined course of therapy or source control. The cohort receiving suppressive antibiotic therapy is typically highly comorbid and the infections often involve retained prosthetic material. In part due to a lack of clear guidelines regarding the use of suppressive antibiotics, and in part due to the complex nature of the infections in question, patients are often prescribed suppressive antibiotics for extremely long, if not indefinite, courses. The risks of prolonged antibiotic exposure in this context are not fully characterised, but they include adverse drug effects ranging from mild to severe, the development of antibiotic resistant organisms and perturbations of the gastrointestinal microbiome. In this narrative review we present the available evidence for the use of suppressive antibiotic therapy in four common indications, examine the gaps in the current literature and explore the known and potential risks of this therapy. We also make suggestions for improving the quality of evidence in future studies, particularly by highlighting the need for a standardised term to describe the use of long-courses of antibiotics to suppress hard-to-treat infections.

Supplementary data
Email alerts, citing articles via, looking for your next opportunity.
- Recommend to your Library
Affiliations
- Online ISSN 1537-6591
- Print ISSN 1058-4838
- Copyright © 2024 Infectious Diseases Society of America
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
NEWS... BUT NOT AS YOU KNOW IT
The photos that expose the horror of Putin’s ‘torture by hunger’

Share this with

A Chernobyl security guard is the latest face of ‘hunger torture’ by Putin’s soldiers, after being held as a prisoner of war in Russia for more than two years.
Gorilyk Roman Vasilyovich was among 168 workers protecting the nuclear facility, who were taken captive and deported from Ukraine through Belarus a few weeks after the start of Putin’s invasion in March 2022.
He was finally returned to his homeland on Friday as part of a POW exchange – but he was never a soldier.
Now pictures of Gorilyk serve as evidence of the Kremlin using hunger as a torture method.

He did get out of alive – barely – but his emaciated condition is reminiscent of the way prisoners in Nazi concentration camps were treated during World War Two.
His skeletal frame is a testament to prolonged deprivation. Gorilyk looks frail. His ribs and bones protrude sharply under his pallid skin.
Muscles have withered away, leaving his limbs fragile, like twigs that could snap.
His face is hollowed, cheeks and eyes sunk deep into his skull. What is left of Gorilyk is skin and bones, and it will take a long time before he recovers.

The pictures were shared online by I Want To Live, a hotline that receives appeals from Russian servicemen in Ukraine.
It said: ‘Gorilyk and the other 168 national guards guarding the Chernobyl plant were literally taken hostage by Russians and deported from Ukraine via Belarus in March 2022.
’89 are still being held captive, and Russia uses them to exchange them for troops captured in battle.
‘The condition of Gorilyk and other Ukrainian prisoners of war is terrifying and associations with the darkest pages of human history – Nazi concentration camps.

‘During their stay in Russian captivity, Roman and other 74 Ukrainian POWs returned by exchange, never visited the observers of the International Committee of the Red Cross.
‘Not allowing observers to prisoners of war in violation of the provisions of the Geneva Conventions is a conscientious and purposeful policy of the Russian government.
‘This is done on purpose so that the International Red Cross cannot record the way the Russian side treats prisoners of war.’
Other Ukrainians have also reported being starved while detained, in addition to being beaten, raped and electrocuted by their genitals.
Get in touch with our news team by emailing us at [email protected] .
For more stories like this, check our news page .
MORE : Passengers thrown from runaway tram before it crashes into another at high speed
MORE : Paranoid Putin ‘starts wearing bulletproof vests for public appearances’
MORE : Tom Cruise, the Paris Olympics and a sinister Russian influence plan
Sign Up for News Updates
Get your need-to-know latest news, feel-good stories, analysis and more.
Privacy Policy

Get us in your feed

IMAGES
COMMENTS
It prevents travelers known or suspected to have a contagious disease that poses a threat to the public's health from boarding commercial airplanes. The Do Not Board list prevents a person from obtaining a boarding pass for any flight into, out of, or within the United States. The Transportation Security Administration (TSA) enforces this list.
CDC uses Travel Health Notices (THNs) to inform travelers about global health risks during outbreaks, special events or gatherings, and natural disasters, and to provide advice about protective actions travelers can take to prevent infection or adverse health effects. A THN can be posted for: 1) a disease outbreak (higher number of expected ...
More. Learn about CDC's Traveler Genomic Surveillance Program that detects new COVID-19 variants entering the country. Sign up to get travel notices, clinical updates, & healthy travel tips. CDC Travelers' Health Branch provides updated travel information, notices, and vaccine requirements to inform international travelers and provide ...
Please see the Travelers' Health site for a complete list. Level 1 - Oropouche Fever in the Americas June 2024. Level 2 - Chikungunya in Maldives May 2024. Level 1 - Global Measles May 2024. Level 2 - Global Polio May 2024. Level 1 - Meningococcal Disease in Saudi Arabia - Vaccine Requirements for Travel During the Hajj and Umrah Pilgrimages ...
Destinations. Measles cases are increasing globally, including in the United States. The majority of measles cases imported into the United States occur in unvaccinated U.S. residents who become infected during international travel. A list of countries with confirmed measles outbreaks can be found on the Global Measles Travel Health Notice (THN).
State Department Travel Advisory Updates. In order to provide U.S. travelers detailed and actionable information to make informed travel decisions, the Department of State regularly assesses and updates our Travel Advisories, based primarily on the U.S. Centers for Disease Control and Prevention (CDC) Travel Health Notices (THNs) and secondary factors such as commercial flight availability ...
The Centers for Disease Control and Prevention (CDC) has issued new COVID-19 travel guidelines for more than 120 countries. The updated advice offers detailed information on specific countries for travelers who are vaccinated and for those who aren't. The CDC specifically assigns risk levels to each country based on the number of cases per ...
All →. Since October 2020, the WHO Guideline Development Group for International Travel and Health (WHO ITH GDG) is tasked with regularly evaluating the scientific evidence around the effectiveness, safety and impact of public health measures for reducing travel-associated spread of SARS-CoV-2, the virus that causes COVID-19, while avoiding ...
Coronavirus disease (COVID-19): Travel advice for the general public Coronavirus disease (COVID-19): Working in hotels and other ... Air travel exposes passengers to a number of factors that may have an impact on health. Some medical conditions and lifestyle choices may affect the safety and comfort of air travel and should be considered before ...
Coronavirus disease (COVID-19): Travel advice for the general public 29 June 2023 | Q&A . Updated 29 June 2023. ... (HEPA) filters, which can remove viruses and germs quickly, minimizing the duration of the exposure to any potentially infectious materials produced by a cough or sneeze. The cabin air system is designed to operate most ...
There are different types of travel-associated infections that cause fever, along with other symptoms to include diarrhea, vomiting, rashes, and muscle aches. The most common of these include malaria, dengue, typhoid fever, and chikungunya. Malaria. Malaria is a parasitic disease spread by mosquitoes in tropical and subtropical climates.
to 21 days) after initial exposure. The virus is transmitted through direct contact with infectious droplets or by airborne spread when an infected person breathes, coughs, or sneezes, and can remain infectious in the air and on surfaces for up to 2 hours after an infected person leaves an area. Individuals infected with
Infectious diseases are disorders caused by organisms — such as bacteria, viruses, fungi or parasites. Many organisms live in and on our bodies. ... Travel wisely. If you're traveling out of the country, talk to your doctor about any special vaccinations — such as yellow fever, cholera, hepatitis A or B, or typhoid fever — you may need. ...
Malaria is the most common febrile illness among travelers to endemic areas and is caused by the mosquito-borne parasites of the Plasmodium genus, primarily P. falciparum, P. vivax, P. ovale, and P. malariae. Cases in the USA have been increasing over the last few decades to over 2000 cases reported to the CDC in 2016.
Abstract. This research explored the role of air travel in the spread of infectious diseases, specifically severe acute respiratory syndrome (SARS), H1N1, Ebola, and pneumonic plague. Air travel provides the means for such diseases to spread internationally at extraordinary rates because infected passengers jump from coast to coast and ...
Vaccination can prevent some diseases. Get your travel vaccinations. Particularly if there's a travel warning for a vaccine-preventable disease where you're going. Ensuring you're vaccinated can protect you and those around you. Talk to your doctor. Ask what travel vaccines they recommend. And find out if your standard vaccinations need boosting.
Abstract. Greater human mobility, largely driven by air travel, is leading to an increase in the frequency and reach of infectious disease epidemics. Air travel can rapidly connect any two points on the planet, and this has the potential to cause swift and broad dissemination of emerging and re-emerging infectious diseases that may pose a ...
Avian Flu (Bird Flu) COVID-19. Chagas Disease (American Trypanosomiasis) Chikungunya. Cholera. Crimean-Congo Hemorrhagic fever. Dengue. Diphtheria. Ebola and Marburg.
Global change, including climate change, urbanization and global travel and trade, has affected the emergence and spread of infectious diseases. In the Review, Baker, Metcalf and colleagues ...
A travel health warning has been issued after a tourist became infected with measles on holiday in Europe and brought it back home ... Measles is one of the most highly infectious diseases and ...
The main infectious diseases to which travellers may be exposed, and precautions for each, are detailed in the Chapter 5 of the International travel and health situation publication.The most common infectious illness to affect travellers, namely travellers' diarrhoea, is covered in Chapter 3 of the International travel and health situation publication.
The CDC told health departments and providers to consider using several of the preferred antibiotics used to treat meningococcal disease in people associated with travel to Mecca.. People who come ...
Latin America is experiencing its worst dengue fever outbreak on record. Case numbers in the first 4 ½ months of 2024 are already 238% higher than they were by this time last year, which itself ...
The deadly disease is highly infectious: 9 out of 10 unvaccinated people will catch it if exposed. About 1 in 5 unvaccinated people who get measles are hospitalized, as many as 1 in 20 kids who ...
HealthDay News — The U.S. Food and Drug Administration is warning parents about a goat milk infant formula potentially tainted with a bacterium that's very dangerous to babies. Crecelac brand formula, already under recall since May 24, could contain Cronobacter, which "can cause bloodstream and central nervous system infections, such as ...
The HSE infectious disease unit boarded the flight at Dublin Airport. Appropriate protocols were enforced by Dublin Airport Operations and Airport Police to ensure the safety of passengers on the ...
Coronavirus disease 2019 (COVID-19) is a respiratory illness caused by the virus SARS-CoV-2. The virus spreads mainly from person to person through respiratory droplets and small particles produced when an infected person coughs, sneezes, or talks. The virus spreads easily in crowded or poorly ventilated indoor settings.
The European Centre for Disease Prevention (ECDP) reported that a staggering 1,671 cases of the potentially fatal disease were recorded in April alone, with Romania, Poland and Belgium being some ...
Corresponding author: Dr Jillian Lau, E: [email protected], F: +61 3 95944533, Ph: +61 3 95944564, Mail: Monash Infectious Diseases, Monash Medical Centre, 246 ...
A Chernobyl security guard is the latest face of 'hunger torture' by Putin's soldiers, after being held as a prisoner of war in Russia for more than two years. Gorilyk Roman Vasilyovich was ...