
- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons

Margin Size
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

26.4B: Sperm
- Last updated
- Save as PDF
- Page ID 8244
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
Sperm are the male “seeds,” germ cells, or gametes.
LEARNING OBJECTIVE
Describe the anatomy and function of sperm
Key Takeaways
- Sperm fertilize the oocyte, donate the paternal chromatin, and provide the centrosome that maintains the zygote’s microtubule system.
- Sperm have three parts: a head, which holds the chromatin, a midpiece filled with mitochondria to provide energy, and a flagellum or tail to move the sperm from the vagina to the oocyte.
- Sperm with one tail, such as human sperm, are referred to as spermatozoa.
- Sperm quality and quantity decrease with age.
- anisogamy : The form of sexual reproduction that involves the union or fusion of two gametes that differ in size and/or form.
- spermatozoa : A motile sperm cell or moving form of the haploid cell that is the male gamete.
- acrosome : A caplike structure over the anterior half of the sperm’s head.
- ATP : An acronym for adenosine triphosphate, which transports chemical energy within cells for metabolism.
- oogamy : A form of anisogamy (heterogamy) in which the female gamete (oocyte) is significantly larger than the male gamete (sperm) and is non-motile. The male gametes are highly motile and compete for the fertilization of the immotile oocyte.
The term sperm is derived from the Greek word for seed and refers to the male reproductive cells. In the types of sexual reproduction known as anisogamy and oogamy, there are marked differences in the size of the gametes, with the smaller termed the “male” or sperm cells. Sperm cells cannot divide and have a limited lifespan. After fusion with egg cells during fertilization, a new organism forms, beginning as a totipotent zygote. The human sperm cell is haploid so that its 23 chromosomes can join the 23 chromosomes of the female egg to form a diploid cell. During fertilization, the sperm provides the following three essential parts to the oocyte:
- A signalling or activating factor that causes the metabolically dormant oocyte to activate
- The haploid paternal genome
- The centrosome, which is responsible for maintaining the microtubule system
Sperm Anatomy

Closeup of Mammalian Fertilization: Micrograph of a sperm poised to enter an ovum
Sperm develop in the testes and consist of a head, a midpiece, and a tail. The head contains the nucleus with densely coiled chromatin fibers, surrounded anteriorly by an acrosome that contains enzymes for penetrating the female egg. The midpiece has a central filamentous core with many mitochondria spiraled around it.
Sperm Physiology and Function
In animals, most of the energy (ATP) for sperm motility is derived from the metabolism of fructose carried in the seminal fluid. This takes place in the mitochondria located in the sperm’s midpiece. This energy is used for the journey through the female cervix, uterus, and uterine tubes.
Motile sperm cells typically move via flagella and require a water medium in order to swim toward the egg for fertilization.These cells cannot swim backwards due to the nature of their propulsion. The uniflagellated sperm cells (with one flagellum) of animals are referred to as spermatozoa.
Human Sperm : Detailed and labeled diagram of a human spermatozoa
Fertility Factors
Sperm quantity and quality are the main parameters in semen quality, a measure of the ability of semen to accomplish fertilization. The genetic quality of sperm, as well as its volume and motility, all typically decrease with age.
All about sperm
The Path of Sperm: A Journey from Testes to Fertilization.

Short answer path of sperm: During ejaculation, sperm travel from the testes through the epididymis, vas deferens, seminal vesicles and prostate gland before being ejaculated out of the penis into the female reproductive tract. Once inside, they swim through the cervix into the uterus and then up to the fallopian tubes where fertilization can occur.
A Step-by-Step Guide to Understanding the Path of Sperm
How does the path of sperm work, frequently asked questions about the path of sperm.
Following the Journey: Tracking the Path of Sperm
The Pathway to Fertilization: Understanding the Journey of Sperm
Decoding the complexities: an in-depth look at the path of sperm.
Table of Contents
When it comes to fertility and conception, understanding the path of sperm can be crucial to having a successful outcome. Sperm is the male reproductive cell in charge of fertilizing the female egg, creating new life. Knowing how this little cell navigates through the numerous obstacles it encounters on its way to meeting its destination can lead to valuable insight for couples struggling with infertility.
In this step-by-step guide, we will break down the journey of sperm from start to finish, outlining each stage in detail so that you can get a better understanding of what happens behind-the-scenes in your reproductive system.
1. Production: The first step in understanding how sperm travels starts with production. Sperm cells are formed inside the testicles within tiny tubes called seminiferous tubules. These cells are manufactured continuously throughout a man’s life span and take around 70 days to mature fully.
2. Maturation: After production is complete, immature sperm cells move out of the testicles and head towards the epididymis, where they continue their maturation process by gaining motility and developing fertilization abilities.
3. Storage: Upon maturation completion, sperm cells remain stored inside tiny ducts located within each epididymis until they receive an ejaculatory signal from nerve endings during sexual intercourse.
4. Emission: When stimulated enough, ejaculation occurs from muscle contractions that transport mature sperm from storage ducts through two long muscular tubes called vas deferens- ultimately reaching their final destination- the urethra.
5. Ejaculation: Once in the urethra- ejaculation occurs- propelling millions of sperm towards their ultimate goal- fertilizing an egg located inside a woman’s reproductive system typically only 30 minutes after release.
6.Lifespan: Once released into a woman’s vagina – most sperms make fail quickly as vaginal acidity inhibits or kills them off almost immediately- however some may survive roughly five days.
Navigating the journey from production to fertilization is not easy, as sperm faces obstacles such as acidity changes in their environments and aggressive mucus.Alongside many other that try to prevent them from reaching their final destination. Even though only one sperm out of millions will successfully fertilize a single egg- with technological advancements and fertility treatments- there is always hope for a successful pregnancy!
Understanding the Path of Sperm can be beneficial to couples dealing with infertility or who want to learn more about how the human reproductive system functions. Hopefully, this guide has allowed you to gain valuable insight into an essential part of reproduction!
The path of sperm may seem like a simple process, but it is actually a highly complex and fascinating journey. For those who have ever wondered how sperm gets from Point A to Point B, here’s a detailed account of the path of sperm:
Step 1: Production
Sperm production starts at the testicles, where millions of them are produced every day. It takes about 74 days for the male body to produce fully mature and functional sperm.
Step 2: Maturation
After being produced in the testicles, immature sperm travel through a tube called the epididymis. This is where they mature and gain mobility over approximately two weeks.
Step 3: Erection
When sexually aroused, blood flows into the penis causing it to become erect. At this point, the urethra (the tube that carries urine out of the body) is also opened up to allow for ejaculation.
Step 4: Ejaculation
During ejaculation, muscles in the pelvic floor contract sending semen (a mixture of fluids secreted by glands in the reproductive system) out of the body through the urethra.
Step 5: Swimmingly along!
Once outside of the male’s reproductive system, an incredible race begins as millions of individual sperms must now make their way through unfriendly territory towards their goal – fertilization.
Step 6: Finding its way
Despite popular belief that every single sperm cell makes its way to an egg on its own accord; In most cases there will be only one winner in this “sprint” with each race primarily consisting usually between several thousand sperms all scrambling forward at breakneck speed to achieve victory over millions others who never made it thus far.
As relatively weak swimmers when compared to other cells across nature due to its size and general physiology; when millions are placed together in such crowded scenarios,it guarantees almost all eggs(whether implanted or otherwise) have numerous sperm cells around them, with usually only one getting through to fertilize..
Step 7: Fertilization and conception
The successful sperm that reaches the egg pulls itself towards the outer layer of the cell wall in order to penetrate, and finally fuse with it thus completing the process of fertilization. Upon fusion, a multitude of biochemical events get triggered from numerous different micro-organs which includes but not limited to release of massive quantity energy together with various enzyme/molecular clashes; ultimately resulting into birth/conception.
In Conclusion;
The path of sperm is truly a remarkable journey – from production to race for competition through swarms and obstacles until achieved fertilization and implantation towards bringing every new human life into existence. It’s mind-blowing to think how many millions of individual cells fight so valiantly just for one successful result- truly Nature’s way demonstrating how tedious yet intriguing life is !
When it comes to reproduction, we often think about the role of the egg, but what about the path of sperm? Sperm plays an essential role in fertilization and conception. In fact, once released by males, sperm remains active for up to 72 hours as they navigate through a complex pathway in search of the egg. Curious about this journey? Here are some frequently asked questions about the path of sperm:
1. What is the path of sperm after ejaculation?
After ejaculation, sperm takes a voyage from the testes through various pathways including the vas deferens and epididymis before entering into the ejaculatory duct located in the prostate gland. Once inside this duct, it journeys out through penis and then penetrates through cervix in females.
2. How long does it take for sperm to reach an egg?
Typically, it takes around 45 minutes for sperms to traverse secondary pathway where each sample contains around 300 million individual sperms. However studies suggest that on average reaching to ovum can take up to three days.
3. Can all sperms reach their destination?
Not all individuals’ sperms successfully make it to their desired destination -the egg! Some get lost along the way while others may have poor quality or not enough energy/motility patterns/sperm count.
4. Why do men produce so many sperms with only one being needed for fertilization?
Sperm competition is real and evolution has programmed male’s bodies accordingly since embryonic development stages itself i.e they keep producing billions of useful and healthy sperms daily primarily because allot will fail during journey/die-off-tried or lost meanwhile still enabling fertilization chances remain high as possible via several eggs that could be potentially successful mates.
5. Can external factors affect the mobility and viability of sperm?
Yes – several environmental hazards such as radiation exposure along with drug abuse/age/stressful activities/poor lifestyle choices affecting general erectile health/mobility/viability of sperm cells reducing fertility or potential impotency.
In summary, the path of sperm is a critical factor in fertilization and conception. Understanding its journey can help us appreciate the complexity of reproductive biology and importance to take care our bodily health and wellbeing that impact our ability to conceive and eventually grow our families.
For millions of couples around the world, conceiving a child is not always an easy feat. The entire process of trying to conceive can be shrouded in mystery and leave many feeling frustrated and confused. However, thanks to modern technology and advancements in scientific research, we now have a better understanding of the journey sperm take on their way to fertilize an egg.
Sperm are microscopic and cannot be seen without the help of powerful microscopes. Thus, tracking their journey has been a challenge for scientists over time. Nonetheless, in recent years, they’ve managed to from start to finish using advanced imaging techniques by bringing light out about complex details that were never understood before.
The journey begins with a male ejaculating semen into his partner’s vagina during intercourse; this semen contains thousands upon thousands of tiny tadpole-like sperm swimming frantically towards their ultimate destination- the fallopian tubes where they will encounter an egg. Along this tumultuous path fraught with multiple obstacles such as acidic bodily fluids/alcohol/smoking or Intense hygiene products/deodorants/bad lifestyle habits that could kill off most weak sperms, only a few dozen viable sperm will make it through alive.
Once inside the female genital tract ,the swim upstream becomes more challenging with vaginal acidity posing greater risk but thanks to various adaptations by these wiggly creatures like tail movements/wriggles forward slingshot/ hyper-acid protection shields and more mucus slurping capabilities., mean those who reach uterus have surpassed almost insurmountable odds.
As ever-moving creatures from ejaculation point probably moving several millimeters per minute now begin sprinting at breakneck speed navigating through twisting passages of cervix/uterus/womb until they reach Fallopian tubes usually covering distances over 10cm.
Finally, within one hour after ejaculation only 50% are left as others become disorienteddeadimmobilized within the vaginal cavity while for sperm that make it to close proximity of the egg they are still not out of the woods as they must overcome several challenges/impenetrable layers in order to attach; successful ones will release enzymes that eat through zona pellucida allowing them to burrow deeper into egg where in 24 hours they form a new life.
In conclusion, there are many obstacles and challenges that sperm face on their journey towards fertilization. However, understanding these challenges has given us greater insight into conception and infertility, allowing us to develop new technologies and treatments that could help millions of couples conceive naturally. So next time you ponder about trying-to-conceive process, give appreciation for this fascinating journey our sperms undertake!
Fertilization is the magical process that brings about new life. And when we talk about fertilization, the first thing that comes to mind is sperm. Sperm cells are like tiny warriors that embark on a perilous journey in search of the ovum. In fact, only one lucky sperm out of millions manages to reach and fertilize the egg, thus beginning a new chapter in human reproduction. But do you know what it takes for a sperm cell to complete this incredible journey? Let’s explore The Pathway to Fertilization: Understanding the Journey of Sperm.
The journey of sperm begins in the testes where millions of them are produced each day. These tiny cells then undergo maturation through a process called spermatogenesis before being released into the epididymis – a coiled tube attached behind each testicle. Here, they go through further maturation before moving up into the vas deferens – another tubular structure responsible for transporting matured sperm from epididymis towards urethra and ultimately outside of male body through ejaculation.
The speed at which these little swimmers travel is extraordinary; with some reports claiming they can swim up to 4mm per minute! However, for most healthy men, it takes several minutes for all existing colonies within ejaculate (semen) to leave their original home base and enter into fallopian tube alongside female’s egg awaiting them.
But why does it take so long? Think of it this way; imagine an army sent on a mission deep into enemy territory that must navigate hazardous terrain while facing numerous obstacles along its path. Similarly, sperm face hurdles as they try to reach the ovum located inside fallopian tubes. They need to fight their way past different obstacles such as acidic pH levels and physical barriers before finally reaching their intended destination.
Once inside fallopian tubes—which are connected by fimbriae or finger-like projections from ovaries—the sperm quickly take advantage of the fluid present around the egg. They swim through towards it, using tail for propulsion and enzymes on their head to dissolve the outer layer surrounding egg . As soon as one lucky sperm penetrates this layer, a chemical reaction occurs that prevents all others from following suit.
But even when a single sperm has crossed the winning line, there are still several hurdles ahead before fertilization can occur. The nucleus of both ovum and sperm merge together, forming what’s known as a zygote – or embryonic cell- initiating a series of cellular events necessary for life to begin.
In conclusion, understanding The Pathway to Fertilization: Understanding the Journey of Sperm can give us a glimpse into how amazing and complex this process is. From their production in testes through maturation in epididymis and vas deferens down into the perilous journey to reach fallopian tubes alive – these little warriors face countless obstacles along their way. But with determination, strength, and agility they make it happen! So next time you see a
The journey of sperm from its origin in the testicles to its final destination in the female reproductive tract is a complex and fascinating process. This journey is essential for ensuring successful fertilization and ultimately, reproduction. Today, we are decoding the complexities of this path with an in-depth look at the path of sperm.
Firstly, let’s start with the basics: Sperm are produced in the seminiferous tubules within the testes. Once sperm have matured, they then travel through a series of ducts within the male reproductive system before exiting through the urethra during ejaculation.
Now that we’ve established where sperm come from, let’s dive deeper into their journey through the male reproductive system. After leaving the seminiferous tubules, sperm move into a small tube called the epididymis where they can be stored for up to several weeks.
If you were thinking that was it, hold your horses because we’re not done yet! The next area that sperm encounter as they move through their complicated journey is called vas deferens. This is a long muscular tube that conveys matured sperm from each epididymis to ejaculatory ducts just behind your bladder.
Once inside these ejaculatory ducts, semen (a mixture composed of both fluids and sperm) will join up with fluids created by other glands along this route – including seminal vesicles, prostate gland or bulbourethral gland – prior to being expelled during ejaculation out through penis.
However, there’s one complication connected to when men undergo vasectomies; since vas deferens transport sperms alongside other particles like minerals dissolved in water too-large-to-pass-through pores- which could hinder successful transportation and possible fertilization using any form natural method or artificial insemination procedure.
Now that we’ve seen how intricate and delicate this whole process can be let us take a closer look at what happens once those little swimmers make it to their destination – the female reproductive tract!
Once inside the female body, sperm will have to face a new set of challenges and obstacles before they can reach the egg. The first hurdle is gaining entry into the cervix, which is a small canal-like structure that connects the uterus with the vagina. This area can be difficult to navigate due to its narrow opening and thick mucus barrier.
If lucky enough and have managed to enter through this main checkpoint, then the next challenge involves finding-and-fertilizing one of several (if not only single) eggs in a region known as Fallopian tubes or oviducts both rolled up like skinny straws extending from each ovary right across abdomen towards opposite side beside uterus wall.
It’s important to note that not all sperm that make it this far will successfully fertilize an egg. Many factors are at play here such as sperm motility (how well they swim), numbers of good-quality swimmers amongst them as well availability of eggs in general.
In conclusion, it’s essential for us to understand how complex and intricate

Sperm's secret voltage switch: Scientists unlock the mystery of motility
Researchers at Stockholm University have unveiled the hidden intricacies of how sperm go from passive bystanders to dynamic swimmers. This transformation is a pivotal step in the journey to fertilization, and it hinges on the activation of a unique ion transporter.
Imagine sperm as tiny adventurers on a quest to reach the ultimate treasure, the egg. They don't have a map, but they make use of something even more extraordinary: chemo-attractants. These are chemical signals released by the egg that act as siren call, directing and activating the sperm. When these signals bind to receptors on the sperm's surface, it triggers a series of events, starting their movement towards the egg. And in this intricate scenario, one key player is a protein known as "SLC9C1."
It's exclusively found in sperm cells, and it is usually not active. However, when the chemo-attractants interact with the sperm's surface, everything changes.
"SLC9C1 operates like a highly sophisticated exchange system. It swaps protons from inside the cell for sodium ions from the outside, temporarily creating a less acidic environment within the sperm. This change in the internal environment triggers increased sperm motility," says David Drew, Professor in Biochemistry at Stockholm University.
The activation of SLC9C1 is driven by a change in voltage that occurs when chemo-attractants attach to the sperm. To accomplish this, SLC9C1 uses a unique feature called a voltage-sensing domain (VSD). Typically, VSD domains are associated with voltage-gated ion channels. But in the case of SLC9C1, it's something truly exceptional in the realm of transporters.
Researchers, led by David Drew, have unveiled the secrets behind SLC9C1's inner workings and provides the first example of voltage-sensing domain activation of a transporter and its connection via an unusually long voltage-sensing (S4) helix.
"The VSD domain responds to the change in voltage by pushing its rodlike S4 helix inwards. This clears the way for ion exchange by SLC9C1, ultimately initiating sperm motility," says David Drew.
"Transporters work very differently than channels and, as such, the VSD is coupled to the sperm protein in a way that we have just never seen before, or even imagined. Its exciting to see how nature has done this and perhaps, in the future, we can learn from this to make synthetic proteins that can be turned-on by voltage or develop novel male contraceptives that work by blocking this protein," David Drew notes.
The research was made possible through funding from the European Research Council (ERC) grant EXCHANGE.
- Pregnancy and Childbirth
- Prostate Cancer
- Nervous System
- Human Biology
- Medical Topics
- Birth control
- Somatic cell
- Fertilisation
- Somatic cell nuclear transfer
- Stem cell treatments
Story Source:
Materials provided by Stockholm University . Note: Content may be edited for style and length.
Journal Reference :
- Hyunku Yeo, Ved Mehta, Ashutosh Gulati, David Drew. Structure and electromechanical coupling of a voltage-gated Na+/H exchanger . Nature , 2023; DOI: 10.1038/s41586-023-06518-2
Cite This Page :
Explore More
- Birdsong Evolution: Tiny NZ Bird
- Sharks Have Depleted Functional Diversity
- Infectious H5N1 Flu Virus in Milk?
- Critical Role of Sleep in Memory Formation
- Preserving DNA in Amber-Like Polymer
- Producing Plasma 'Fireballs' On Earth
- Mixed Emotions Are Real
- Rotation of Earth's Inner Core Recently Slowed
- Nanosized Blocks Self-Assemble Into ...
- Dolphins With Elevated Mercury Levels
Trending Topics
Strange & offbeat.
- Meet the Mentors
- Get Involved
- Get the T-Shirt
- Life Science Marketing
- Community Marketing
- Custom Marketing
Join Us Sign up for our feature-packed newsletter today to ensure you get the latest expert help and advice to level up your lab work.
- Genomics & Epigenetics
- DNA / RNA Manipulation and Analysis
- Protein Expression & Analysis
- PCR & Real-time PCR
- Flow Cytometry
- Microscopy & Imaging
- Cells and Model Organisms
- Analytical Chemistry and Chromatography Techniques
- Chemistry for Biologists
- Basic Lab Skills & Know-how
- Equipment Mastery & Hacks
- Managing the Scientific Literature
- Career Development and Networking
- Dealing with Fellow Scientists
- Getting Funded
- Lab Statistics & Math
- Organization & Productivity
- Personal Development
- PhD Survival
- Soft Skills & Tools
- Software & Online Tools
- Survive & Thrive
- Taming the Literature
- Writing, Publishing & Presenting
- History of Biology
A Short History of Cell Biology
Our journey to understanding that single cells are the fundamental units of life traces back to groundbreaking scientific milestones, such as the invention of the microscope, which revealed individual cells, and advancements like the discovery of fluorescent proteins and electron microscopes that have enriched our insights into the intricate structure and function of cells. Dive into a short history of cell biology.
Published October 9, 2023

Having earned both a PhD and an MBA, Dan is uniquely qualified to understand the medical and financial needs in the insurance industry. He is a successful consultant, connecting clients with the financial products most suited to their needs. He specializes in private health insurance, private life insurance, dental, vision, Medicare supplement, indexed annuities, and international health insurance.
Listen to one of our scientific editorial team members read this article. Click here to access more audio articles or subscribe.
The defining feature of cell theory is that single cells are the fundamental unit of life and can exist alone or combine to form multicellular organisms. The history of cell biology and the formation of cell theory involved several key developments and discoveries, including the invention of the compound microscope in 1595, the visualization of cells in cork by Robert Hooke in 1655, and the visualization of live cells under the microscope by Anton van Leeuwenhoek in 1674.
In modern cell biology, we know that single cells are the fundamental unit of life and can exist as single cells (unicellular organisms) or combine to form multicellular organisms. But how did we reach this understanding? Let’s dive into a short history of cell biology.
A Quick Refresher on the Structure of Cells
Before we get started on the history of cell biology, let’s have a quick refresher on the basic structure of individual cells.
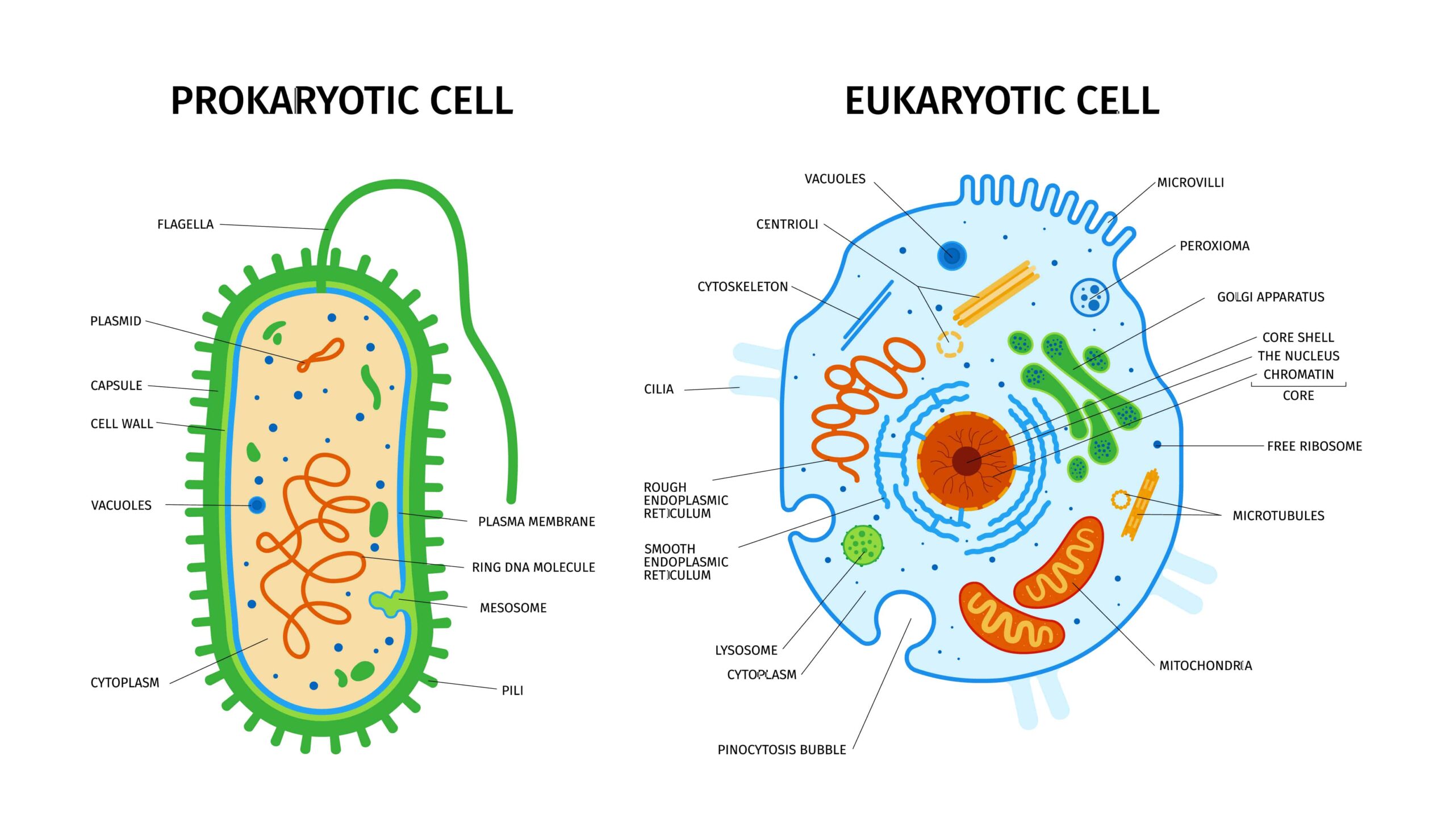
Cells come in various types, from prokaryotic cells, such as bacteria and archaea, to eukaryotic plant and animal cells. Within these groups, there are further distinct cell types, such as red blood cells, neurons, and epithelial cells.
These distinct cell types vary in their structures, depending on their cell specialization. However, cell membranes are a defining feature of cells. These are required to maintain a fixed environment within the cell. They regulate the movement of chemicals across the membrane both in and out of the cell.
Most cells also contain genetic material in the form of deoxyribonucleic acid (DNA). In eukaryotes, DNA is stored within a subcellular compartment known as the nucleus.
In prokaryotes, there are no intracellular membranes, and the DNA is located in the cytoplasm. Some types of cells with specific functions lack DNA, such as mature red blood cells. Figure 1 shows the general structure of prokaryotic and eukaryotic cells.
Cell Theory
The cell theory, or cell doctrine, states that all organisms are composed of similar basic units of organization called cells. The concept was formally articulated in 1839 by Schleiden & Schwann and has remained as the foundation of modern biology. The idea predates other great paradigms of biology, including Darwin’s theory of evolution (1859), Mendel’s laws of inheritance (1865), and the establishment of comparative biochemistry (1940).
First Cells Seen in Cork
While the invention of the telescope made the Cosmos accessible to human observation, the light microscope opened up smaller worlds, showing what living forms were composed of. The cell was first discovered and named by Robert Hooke in 1665. He remarked that it looked strangely similar to cellula or small rooms which monks inhabited, thus deriving the name.
However, what Robert Hooke actually saw was the dead cell walls of plant cells (cork) as they appeared under the microscope. Hooke’s description of these cells was published in Micrographia . The cell walls observed by Hooke did not indicate the nucleus and other organelles found in most living cells .
The first man to witness a live cell under a microscope was Anton van Leeuwenhoek , who, in 1674, described the algae Spirogyra. Van Leeuwenhoek probably also saw bacteria.
Formulation of the Cell Theory
In 1838, Theodor Schwann and Matthias Jakob Schleiden were enjoying after-dinner coffee and talking about their studies on cells. It has been suggested that when Schwann heard Matthias Schleiden describe plant cells with nuclei, he was struck by the similarity of these plant cells to animal cells he had observed in tissues.
The two scientists went immediately to Schwann’s lab to look at his slides. Schwann published his book on animal and plant cells (Schwann 1839) the next year, a treatise devoid of acknowledgments of anyone else’s contribution, including that of Schleiden (1838). He summarized his observations into three conclusions about cells:
- The cell is the fundamental unit of structure, physiology, and organization in living things.
- The cell retains a dual existence as a distinct entity and a building block in the construction of organisms.
- Cells form by free-cell formation, similar to the formation of crystals (spontaneous generation).
We know today that the first two tenets are correct, but the third is clearly wrong. The correct interpretation of cell formation by division was finally promoted by others and formally enunciated in Rudolph Virchow’s powerful dictum, Omnis cellula e cellula ,: “All cells only arise from pre-existing cells”.
Modern Cell Theory
- All known living things are made up of cells.
- The cell is the structural & functional unit of all living things.
- All cells come from pre-existing cells by division. (Spontaneous Generation does not occur).
- Cells contain hereditary information, which is passed from cell to cell during cell division.
- All cells are basically the same in chemical composition.
- All energy flow (metabolism & biochemistry) of life occurs within cells.
As with the rapid growth of molecular biology in the mid-20th century , cell biology research exploded in the 1950s. It became possible to maintain, grow, and manipulate cells outside of living organisms .
The first continuous cell line to be so cultured was in 1951 by George Otto Gey and coworkers, derived from cervical cancer cells taken from Henrietta Lacks, who died from her cancer in 1951. The cell line, which was eventually referred to as HeLa cells , has been the watershed in studying cell biology in the way that the structure of DNA was the significant breakthrough of molecular biology.
In an avalanche of progress in the study of cells, the coming decade included the characterization of the minimal media requirements for cells and the development of sterile cell culture techniques. It was also aided by the prior advances in electron microscopy, and later advances such as the development of transfection methods, the discovery of green fluorescent protein in jellyfish, and the discovery of small interfering RNA (siRNA), among others.
The study of the structure and function of cells continues today in a branch of biology known as cytology. Advances in equipment, including cytology microscopes and reagents, have allowed this field to progress, particularly in the clinical setting.
The History of Cell Biology Timeline
Below is a timeline of some of the key events in the development of cell theory and cell biology.
1595 – Jansen is credited with the first compound microscope. 1655 – Hooke described ‘cells’ in cork. 1674 – Leeuwenhoek discovered protozoa. He saw bacteria some nine years later. 1833 – Brown described the cell nucleus in cells of the orchid. 1838 – Schleiden and Schwann proposed cell theory. 1840 – Albrecht von Roelliker realized that sperm cells and egg cells are also cells. 1856 – N. Pringsheim observed how a sperm cell penetrated an egg cell. 1858 – Rudolf Virchow (physician, pathologist, and anthropologist) expounds his famous conclusion: omnis cellula e cellula , that is, cells develop only from existing cells (cells come from preexisting cells). 1857 – Kolliker described mitochondria. 1879 – Flemming described chromosome behavior during mitosis. 1883 – Germ cells are haploid, chromosome theory of heredity. 1898 – Golgi described the Golgi apparatus. 1938 – Behrens used differential centrifugation to separate nuclei from cytoplasm. 1939 – Siemens produced the first commercial transmission electron microscope. 1952 – Gey and coworkers established a continuous human cell line. 1955 – Eagle systematically defined the nutritional needs of animal cells in culture. 1957 – Meselson, Stahl, and Vinograd developed density gradient centrifugation in cesium chloride solutions for separating nucleic acids. 1965 – Ham introduced a defined serum-free medium. Cambridge Instruments produced the first commercial scanning electron microscope. 1976 – Sato and colleagues publish papers showing that different cell lines require different mixtures of hormones and growth factors in serum-free media. 1981 – Transgenic mice and fruit flies are produced. Mouse embryonic stem cell line established. 1995 – Tsien identifies a mutant of GFP with enhanced spectral properties. 1998 – Mice are cloned from somatic cells. 1999 – Hamilton and Baulcombe discovered siRNA as part of post-transcriptional gene silencing (PTGS) in plants. 2006 – Factors required to create induced pluripotent stem cells are identified, allowing stem cells to be created from differentiated cells. 2009 – Single-cell sequencing makes its debut, allowing insight into transcriptomics at the resolution of individual cells.
2009 – First paper published using organoids derived from a single adult stem cell. 2012 – CRISPR gene editing is developed, allowing precise RNA-targetted genome engineering.
A History of Cell Biology Summarized
In the history of cell biology, there have been many individual scientific discoveries and technological developments, from the invention of the microscope, allowing us to see individual cells, to the discovery of fluorescent proteins and the invention of powerful electron microscopes, allowing us to study the function and structure of cells in greater detail.
Nowadays, the availability of microscopes means that most people can now see cells for themselves. Read our article on how to turn a mobile phone into a simple microscope to appreciate how accessible the cellular world is.
Originally published November 2007. Reviewed and updated October 2023.
Further Reading on the History of Cell Biology
- Landmark Papers in Cell Biology : Selected Research Articles Celebrating Forty Years of The American Society for Cell Biology. 2000. Cold Spring Harbor Laboratory Press.
- Mazzarello P. A unifying concept: the history of cell theory. Nat Cell Biol. 1999. 1(1):E13-5.
Share this article:
More 'History of Biology' articles
Southern (blot) exposure remains a useful technique
At a meeting recently, I asked two PhD molecular biologists about the last time they used a Southern blot. After nearly a minute of unrestrained laughter, they asked “Who on earth still does that?” “Maybe for a very, very specific use,” conjectured one of the scientists. When I asked the scientist who taught me the…

How Plasmids Became Embroiled in The Cold War
The humble plasmid. We now know it so well, but as little as 60 years ago the field of extra-chromosomal heredity was decidedly murky. Not only was it the subject of great debate, conflict and friction within the scientific community, it was even used as a politico-religious tool during the Cold War! The origin of…
133 Comments
Having bundle of informations ……plzzzz carry on this type of articles are very precious to us
Leave a Reply Cancel reply
You must be logged in to post a comment.
This site uses Akismet to reduce spam. Learn how your comment data is processed .
Raise your Research Game with Bitesize Bio
Sign up for our feature-packed newsletter today to ensure you get the latest expert help and advice to level up your lab work.
You’ll stay up-to-date with our podcasts, webinars, workshops, downloadables, and more, delivered to your inbox every fortnight.
Don’t delay! Sign up now
Newsletters
All emails contain an unsubscribe link. You can review our privacy policy , cookie policy and terms and conditions online.
- Technical Skills
- More Skills
Bitesize Bio Powered
- Microscopy Focus
- Privacy Policy
- Cookie Policy
- Terms of Use
Copyright © Science Squared – all rights reserved
‘Spermageddon’ Directors Tommy Wirkola, Rasmus Sivertsen Debut Clip: ‘It’s ‘Inside Out’ for Adults; How the Hell Did We Get This Made?!’ (EXCLUSIVE)
By Marta Balaga
Marta Balaga
- Henry Selick Makes Surprise Appearance at Variety’s Annecy Panel on Laika, Talks ‘Ancient Magic’ of Stop-Motion and Remastered ‘Coraline’: ‘It’s Gorgeous’ 2 days ago
- ‘Spermageddon’ Directors Tommy Wirkola, Rasmus Sivertsen Debut Clip: ‘It’s ‘Inside Out’ for Adults; How the Hell Did We Get This Made?!’ (EXCLUSIVE) 2 days ago
- Pyramide Films Boards Stop-Motion Feature ‘Olivia and the Invisible Earthquake’ About a Family Evicted From Their Home (EXCLUSIVE) 3 days ago

As Annecy premiere “ Spermageddon ” continues to rack up sales, even its makers are surprised they got away with its risqué subject matter.
“When we finally got to make it, there were times when I was looking at the screen, thinking: ‘How the hell did we get this movie made?!’” said Tommy Wirkola , who co-directed with Rasmus A. Sivertsen .
“We made it in Europe – that’s how. Years ago, I pitched it in Hollywood and it never went anywhere. When it comes to sex, it’s more delicate over there.”
Related Stories
The ad market is not ready for the imminent streaming sports boom, charli xcx launches an exhilarating new chapter of pop with the innovative ‘brat’: album review, popular on variety.
“It’s cute, it’s awkward. I used to listen to this radio show where people asked the host about sex. It was surprising how little they knew sometimes. We wanted to have this innocence but also reflect the world where you have access to all sorts of sexual imagery and pornography,” added Wirkola.
“I remember watching [Albert Barillé’s] ‘Once Upon a Time… Life.’ It was very matter-of-fact: nothing was disgusting or scary. We tried to do the same. The only note we got from our distributors was: ‘Don’t show any nipples.’ Which was… Absurd.”
For Wirkola, best known for Nazi zombie attacks in “Dead Snow” and for Santa Claus fighting mercenaries in “Violent Night,” animation was unfamiliar ground.
“I brought ‘Spermageddon’ to the producers at 74 Entertainment, and they suggested Rasmus, who obviously made amazing animated films. I jumped at the chance because I didn’t know what the hell I was doing,” he laughed.
“Funnily enough, we never talked about whether we can make it more shocking. We talked about whether we could make it sweeter and more relatable. We wanted to demystify sex and having sex for the first time. It’s normal for it to feel awkward.”
Still, what happens inside of the body is a whole different ball game, with every sperm cell trying its best to succeed.
“We decided to approach the world of humans in a more realistic way – even the ‘acting’ is toned down. Inside of the body, we go crazy. It’s almost a parody of our own reality, where everyone is fighting to be the best and the fastest,” said Sivertsen.
“It was important for people to be able to tell [the sperm cells] apart. At first, we tried to be subtle. Then we just went for it, big time,” added Wirkola. The team also decided to add some outrageous musical numbers.
“With my co-writers Geir Vegar Hoel and Jesper Sundnes, we are big ‘South Park’ fans. I never had a desire to make a musical; it wasn’t in my movie DNA, but then it started to make a lot of sense. Now, these are my favorite moments.”
Despite humor and storylines that push limits, “Spermageddon” also makes safe sex and pro-choice arguments.
“When we wrote the script, it wasn’t that controversial. Then it suddenly became a ‘thing,’ not just in the US but also in many places in Europe. In Norway, no one will even blink,” noted Wirkola.
“I am proud of it, and I am proud of that ending and the fact that despite it being silly, we are also saying something important. It’s not just fun and games: this feels real for these characters.”
“Something I always like to play with is tone, and it feels like so many things at once. It’s pushing the limits, and it has a very big heart. If I were a teenager again, I would love to sneak into the screening and laugh my ass off. We wanted to make something that can’t be put in just one box.”
More from Variety
Korea box office: ‘if’ and other new releases make little impact as ‘the roundup: punishment’ hits $75 million, virtual production market grows as average stage size contracts, theater owners chief on box office downturn, alamo drafthouse sale and bringing audiences back to the movies: ‘we’re still looking for a catalyst’, korea box office: ai fantasy ‘wonderland’ takes weekend win as ‘bad boys: ride or die’ stalls on first lap, live music blues: are black keys, jennifer lopez just the beginning, korea box office: ‘furiosa’ holds soft second weekend lead over ‘the plot’, more from our brands, niall horan surprises fan who manifested sold-out msg show with front row seats, old forester dropped a new batch of its most coveted single-barrel bourbon, mlb’s rules on gambling: what happens when players bet, the best loofahs and body scrubbers, according to dermatologists, nbc orders more episodes of new fall comedy st. denis medical, verify it's you, please log in.
- Open access
- Published: 14 June 2024
Emerging trends in sperm selection: enhancing success rates in assisted reproduction
- Xiang Zhang 1 na1 ,
- Shuen Chao 2 na1 ,
- Ningxin Ye 2 , 4 &
- Dongfang Ouyang 2 , 3
Reproductive Biology and Endocrinology volume 22 , Article number: 67 ( 2024 ) Cite this article
Metrics details
This comprehensive review explores the evolving landscape of sperm selection techniques within the realm of Assisted Reproductive Technology (ART). Our analysis delves into a range of methods from traditional approaches like density gradient centrifugation to advanced techniques such as Magnetic-Activated Cell Sorting (MACS) and Intracytoplasmic Morphologically Selected Sperm Injection (IMSI). We critically assess the efficacy of these methods in terms of sperm motility, morphology, DNA integrity, and other functional attributes, providing a detailed comparison of their clinical outcomes. We highlight the transition from conventional sperm selection methods, which primarily focus on physical characteristics, to more sophisticated techniques that offer a comprehensive evaluation of sperm molecular properties. This shift not only promises enhanced prediction of fertilization success but also has significant implications for improving embryo quality and increasing the chances of live birth. By synthesizing various studies and research papers, we present an in-depth analysis of the predictability of different sperm selection procedures in ART. The review also discusses the clinical applicability of these methods, emphasizing their potential in shaping the future of assisted reproduction. Our findings suggest that the integration of advanced sperm selection strategies in ART could lead to more cost-effective treatments with reduced duration and higher success rates. This review aims to provide clinicians and researchers in reproductive medicine with comprehensive insights into the current state and future prospects of sperm selection technologies in ART.
The field of Assisted Reproductive Technology (ART) has experienced significant transformation with the advent of advanced sperm selection techniques [ 1 , 2 , 3 ]. This section thoroughly explores various innovative methods crucial in gamete and embryo selection, substantially advancing fertility treatments [ 4 , 5 , 6 , 7 , 8 ]. Techniques such as microfluidic sperm sorting, Magnetic-Activated Cell Sorting (MACS) [ 6 , 8 , 9 ], electrophoretic sperm selection, Intracytoplasmic Morphologically Selected Sperm Injection (IMSI) [ 10 , 11 , 12 ], and sperm DNA fragmentation analysis [ 13 , 14 , 15 ] are pivotal in reshaping the ART landscape. They address essential aspects of gamete quality evaluation and selection [ 4 , 6 , 10 , 14 , 15 ]. These subsections offer detailed insights into the benefits, limitations, and developmental trajectory of each method, clarifying their roles in improving reproductive outcomes [ 8 , 10 , 13 , 14 , 15 ]. Additionally, a comparative analysis provides a comprehensive view of traditional and advanced sperm selection methods, underscoring the transformative impact of these innovative approaches [ 4 , 7 , 8 , 10 , 14 ]. Moreover, this section delineates future directions and implications, spotlighting ongoing advancements poised to revolutionize ART, with a focus on more personalized, accessible, and ethically responsible fertility treatments [ 3 , 10 , 14 , 15 , 16 ].

Advanced sperm selection techniques
Microfluidic sperm sorting.
Microfluidic sperm selection techniques have emerged as advanced methods for isolating and sorting motile spermatozoa based on their functionality and morphology. These techniques utilize microfluidic devices that are fabricated using materials such as polydimethylsiloxane (PDMS), a silicon-based organic polymer. The devices consist of microchannels with specific dimensions tailored to the size of sperm cells. Creating flow conditions within the microchannels allows for the separation and collection of motile and morphologically normal spermatozoa, while non-motile spermatozoa and debris exit through a separate outlet(Fig. 1 ) [ 1 , 2 , 3 , 17 ].

Microfluidics Sperm Sorting Pathway: A Visual Overview
During the sorting process of sperm selection through microfluidic techniques, semen samples are introduced into the microfluidic devices. To isolate motile spermatozoa, different strategies are employed depending on the device design. These strategies may involve the use of parallel streams with varying widths, allowing motile spermatozoa to deviate into one stream while non-motile spermatozoa and debris continue along their initial streamlines. Other devices may utilize microporous membranes or precise flow control to separate motile spermatozoa from other components of the semen sample.
Microfluidic sperm selection techniques offer several advantages over conventional methods such as density gradient centrifugation and swim-up techniques (Table 1 ). These microfluidic techniques provide high selectivity and specificity in isolating motile and morphologically normal spermatozoa, resulting in improved sperm selection processes. Additionally, microfluidic devices enable real-time monitoring and analysis of sperm cells, allowing for precise selection based on parameters such as motility, morphology, and DNA integrity. The small-scale nature of microfluidic devices also allows for reduced sample volumes which improv handling of individual sperm cells.
However, it is important to note that microfluidic sperm selection techniques also possess limitations, including manipulation requirements, the potential of device clogging, and the cost of the device. One limitation is the complexity of device fabrication and the requirement for specialized equipment and expertise. The fabrication process involves precise control over microchannel dimensions and the integration of microfluidic components, thereby posing challenges in terms of scalability and accessibility. Another limitation is the potential for device clogging or blockage due to the presence of debris or non-motile spermatozoa in the semen sample. While microfluidic devices aim to separate motile spermatozoa from other components, the presence of debris or non-motile spermatozoa can adversely affect the sorting efficiency and accuracy of the technique. Furthermore, the cost associated with microfluidic sperm selection techniques may be higher compared to conventional methods. The fabrication of microfluidic devices along with the requirement for specialized equipment and materials contribute to the overall cost, which may limit their widespread adoption in certain settings or regions with limited resources.
In summary, microfluidic sperm selection techniques offer advanced capabilities for sperm selection in assisted reproductive technology. These techniques afford high selectivity and real-time analysis of sperm parameters, thereby leading to the enhanced quality of sperm. However, limitations such as the complexity of device fabrication, potential for device clogging, and high cost should be considered. The intricacy involved in the fabrication of devices for microfluidic sperm selection arises from the need for precise engineering and manufacturing processes. Fabricating microchannels with specific dimensions and integrating microfluidic components necessitate specialized expertise and equipment, often requiring cleanroom facilities. The intricate design and assembly process can be time-consuming and challenging, which may hinder widespread adoption, particularly in settings with limited resources where access to advanced fabrication techniques is limited.
One of the critical limitations of microfluidic sperm selection techniques is the potential for device clogging or blockage during the processing of semen samples. The microfluidic devices depend on the controlled fluid flow through microchannels to sort and isolate spermatozoa based on their motility and morphology. However, the presence of debris, non-motile spermatozoa, or other particulate matter in the semen sample may obstruct the microchannels, thereby compromising the accuracy and efficiency of the sperm sorting process. To mitigate this issue, researchers have been actively working on innovative solutions to prevent device clogging and enhance the performance of microfluidic platforms. For instance, Venugopal et al. [ 18 ] introduced a microfluidic platform with an array of uniquely designed multifunctional microposts to achieve higher capture efficiency and flow rates, while effectively avoiding clogging issues. They employed an alternative carry-forward path that allowed particles to bypass congested areas, mitigating the detrimental effects of surge pressure build-up and shear stress on cell viability.
Another approach to addressing clogging concerns is the bioinspired lobe filter system developed by Clark and San-Miguel [ 19 ]. Inspired by the filtration mechanism of manta rays, their microfluidic lobe filters enable efficient filtration of particles in the range of 10–30 μm with precise control and high throughput. The filtration efficiency increased with fluid flow rate, thereby highlighting the role of particle inertial effects in lobe filter separation. These innovations promise to significantly improve the reliability and performance of microfluidic sperm selection techniques, making them more suitable for high-throughput and continuous applications in assisted reproductive technology. In summary, while clogging remains a limitation in microfluidic sperm selection, ongoing research and the development of novel microfluidic designs, such as those inspired by nature and incorporating alternative carry-forward paths, demonstrate significant potential in overcoming this challenge and enhancing the effectiveness of microfluidic sperm sorting for ART.
The exorbitant price associated with microfluidic sperm selection techniques can pose financial challenges for healthcare facilities and patients. The fabrication of microfluidic devices requires specialized materials and equipment, thus contributing to their initial expenses. Additionally, the need for skilled personnel and proper training escalates the overall cost. While the potential benefits of improved sperm quality and ART outcomes are significant, cost considerations may hinder the widespread adoption of these techniques, particularly in regions with limited resources.
To surmount these limitations and fully connect the potential of microfluidic sperm selection techniques in assisted reproductive technology, a synergistic approach involving researchers, clinicians, and industry partners is imperative. Such collaborative endeavors can drive progress in multiple key domains, enhancing the accessibility, efficacy, and cost-effectiveness of these nascent technologies. Researchers could concentrate on simplifying device fabrication processes and refining device designs to mitigate the complexity and cost concerns. Additionally, explorations into novel sample preparation methods can ameliorate the risk of device clogging, ensuring more reliable and consistent results. Clinicians occupy a pivotal role in orchestrating extensive clinical trials to validate the long-term efficacy and impact of microfluidic sperm selection techniques on ART success rates. Collaboration with industry partners, strides in manufacturing technologies and materials can be actualized, thereby potentially diminishing the overall financial burden associated with microfluidic devices and facilitating their affordability for a broader range of healthcare facilities and patients. Through such collaborative endeavors, the field of assisted reproductive technology stands to make substantial progress, thereby extending personalized and effective fertility treatments to couples on a global scale.
Magnetic-activated cell sorting (MACS)
Magnetic-Activated Cell Sorting (MACS) has emerged as a leading technology with significant potential in sperm sorting for ART. This technology functions by utilizing magnetic microbeads coated with specific antibodies to isolate target cells based on their surface markers. In the context of sperm selection, Annexin-V, a calcium-dependent phospholipid-binding protein, is frequently used as the specific antibody to identify apoptotic spermatozoa [ 4 , 5 , 6 , 7 , 20 , 21 , 22 , 23 , 24 ]. Annexin-V binds to phosphatidylserine (PS), a phospholipid typically confined to the inner leaflet of the plasma membrane in viable cells(Fig. 2 ). Nonetheless, during the process of apoptosis, PS is externalized to the outer leaflet of the plasma membrane, thus allowing its detection and binding by Annexin-V. Comprehensive research on this technique has yielded valuable insights into its effectiveness and potential clinical applications across varied patient demographics(statistics that describe populations and their characteristics).

Magnetic-Activated Cell Sorting (MACS): A Visual Overview
The employment of MACS in sperm preparation for ART offers notable advantages, including the selective isolation of viable sperm characterized by reduced DNA fragmentation and enhanced genetic integrity. Such attributes have the potential to augment both fertilization and pregnancy rates (Table 1 ). Studies have demonstrated the efficiency of MACS in ameliorating sperm parameters, ranging from motility and morphology to chromatin integrity, not only in normozoospermic patients but also in those presenting with suboptimal semen parameters [ 7 , 22 , 24 ]. The synergistic use of MACS with density gradient centrifugation (DGC) has shown particularly promising results, leading to a substantial decline in apoptotic spermatozoa and an increase in sperm quality [ 6 , 22 , 24 ]. Furthermore, the integration of DGC-MACS has been associated with superior ART cycle parameters, encompassing diminished sperm DNA fragmentation rates and curtailed oxidative stress, potentially leading to increased success rates in Intracytoplasmic Sperm Injection (ICSI) cycles [ 20 ].
However, certain limitations should be acknowledged (Table 1 ). While MACS has demonstrated encouraging outcomes within specific patient groups, its integration into ART remains nascent. Further research is required to corroborate its efficacy and to buttress the evidence advocating its inclusion in routine sperm selection protocols [ 20 ]. Additionally, the technical intricacy inherent to MACS systems could pose obstacles to its ubiquitous acceptance in clinical environments. Such technical complexity of MACS systems can be attributed to the intricate process involved in isolation of target cells based on their surface markers, utilizing magnetic microbeads and designated antibodies. This protocol mandates exacting calibration and refinement to guarantee precise and efficacious spermatozoa sorting. Moreover, the apparatus and reagents used in MACS procedures necessitate rigorous maintenance and quality assurance, amplifying the overall intricacy of assimilating this technology within clinical laboratories. Consequently, dedicated training and proficiency became indispensable for laboratory personnel to adeptly conduct MACS, potentially constraining its pervasive acceptance in routine clinical practices.
The utilization of MACS in sperm preparation for ART presents a promising approach to augment sperm quality and thereby ameliorate fertility outcomes across a spectrum of patient populations. When amalgamated with DGC, MACS has evinced notable efficacy in the exclusion of apoptotic spermatozoa, favoring the selection of viable sperm characterized by reduced DNA fragmentation, which subsequently bolsters the probability of successful fertilization and elevated pregnancy rates. Such pioneering revelations fortify the expanding corpus of evidence advocating the integration of MACS in tailored sperm selection techniques addressing male factor infertility challenges. An exhaustive appraisal of sperm quality, undertaken via various assays, inclusive of evaluations pertaining to viability, motility, chromatin integrity, and the acrosome reaction, offers an encompassing perspective on sperm functionality and genetic integrity. This rigorous analysis is pivotal for precision-driven sperm selection and the ensuing success of ART, more so in cases marked by abnormal semen parameters or idiopathic infertility. The incorporation of MACS under such circumstances underscores its potential in revolutionizing fertility treatment strategies, thereby offering hope to couples facing challenges in conception.
With the relentless progression of reproductive medicine, further research and validation studies are warranted to not only solidify these previous findings but also to probe the potential expended applications of MACS in ART. An augmented, multi-centric study encompassing a broad spectrum of patient cohorts is pivotal to bolster the empirical evidence supporting MACS’ efficacy, thereby facilitating its integration into routine sperm selection protocols in clinical settings. The adoption of innovative approaches like MACS in ART has the potential to transform the landscape of fertility treatment, exemplifying personalized care and amplifying reproductive success for a on a global scale.
Electrophoretic sperm selection
Electrophoretic sperm selection emerges as a revolutionary modality within the realm of assisted reproductive technologies, capitalizing on the inherent electrical attributes of spermatozoa to segregate functionally adept cells [ 8 , 25 ]. This technique exploits the distinctive electrical charge acquired by sperm throughout their maturation process - a characteristic that is instrumental in preventing aggregation, circumventing nonspecific binding, and mitigating undesired storage within the female reproductive tract [ 8 ]. Over time, considerable progress has been achieved in this field, leading to the refinement of methodologies that aim to ensure sample integrity, reduce DNA damage, and augment overall fertility potential [ 13 , 23 , 24 ]. This section delves into the advantages, limitations, addressed concerns, and future prospects of electrophoretic sperm selection.
The advent of electrophoretic sperm selection heralds a series of noteworthy advantages in the realm of assisted reproduction (Table 1 ). The approach introduces a non-invasive and rapid means of isolating spermatozoa with optimal fertilization potential. The CS-10 device pioneered this technique by capitalizing on the negatively charged attribute of mature sperm, resulting in the birth of viable offspring devoid of embryonic development problems [ 13 ]. The advancement embodied by the Felix™ apparatus further accentuates this advantage by incorporating a filtration system that effectively segregates contaminating cells. This results in the heightened purity and superior quality of isolated sperm populations [ 23 ]. Nevertheless, like any technological advancement, electrophoretic sperm selection presents certain limitations (Table 1 ). A primary challenge resides in maintaining a nuanced equilibrium between sperm membrane charge and functional attributes. Although the exclusion of negatively charged sperm through DGC may enhance DNA damage levels, the intricate dynamics of this process warrant further exploration. Moreover, the formulation of an optimized electrophoretic buffer should be a focal consideration during isolation procedure [ 14 ]. The composition and properties of the buffer critically influence the migration of spermatozoa, dictated by their charge, size, and various other determinants. Suboptimal buffer conditions could potentially lead to inconsistent results and compromised sperm quality during the separation process. The conductivity, pH, and ionic strength of the buffer necessitate rigorous optimization to ensure the precise and effective migration of sperm cells. Moreover, the constituents of the buffer should be carefully selected to mitigate potential detrimental impacts on sperm viability and function. Consequently, a comprehensive investigation of buffer formulations is essential to address this potential limitation, ensuring enhanced consistency, reliability, and reproducibility.
Additionally, the robustness of the technique across a diverse spectrum of semen samples, particularly from pathological donors, requires validation [ 24 ]. Thus, while electrophoretic sperm selection offers considerable potential, it is crucial to address these limitations to ascertain its clinical applicability.
The evolution of electrophoretic sperm selection epitomizes the dynamic nature of scientific progress in addressing pertinent concerns. The CS-10 device introduced the concept of charge-based sperm selection, revolutionizing the field and laying the foundation for subsequent advancements [ 13 ]. The Felix™ device, with its amalgamation of electrophoretic separation and a sophisticated filtration system, stands as a testament to the continual refinement of the technique. This innovation notably addresses the pivotal issue of sample purity by effectively eliminating contaminating cells [ 23 ]. Additionally, Simon et al. (2016) deepened our understanding of sperm membrane charge dynamics, highlighting the intricate relationship between charge and DNA integrity [ 26 ]. Furthermore, Ainsworth et al.‘s (2005) electrophoretic system presents a novel approach to sperm isolation, demonstrating its potential to mitigate DNA damage and enhance functional attributes [ 27 ]. The collective progress made in addressing these concerns underscores the trajectory of electrophoretic sperm selection.
The trajectory of advancements in electrophoretic sperm selection has been significant, showing great potential for the field of assisted reproduction. The transition from CS-10 to the Felix™ device exemplifies a proactive approach to addressing challenges and enhancing the efficacy of the technique [ 13 , 23 ]. The studies by Simon et al. (2016) and Ainsworth et al. (2005) offer a comprehensive insight into the complex dynamics of sperm membrane charge and its implications for fertility potential [ 26 , 27 ]. Additionally, these studies emphasize the importance of rigorous validation across a variety of semen samples to confirm the clinical applicability of this technique. The development of electrophoretic sperm selection is noteworthy, highlighting the capability of innovative approaches to redefine existing paradigms. As progress ensues, the field stands on the cusp of revolutionizing assisted reproduction by leveraging the electrical attributes of sperm for enhanced selection. Nevertheless, the path forward necessitates meticulous validation and comprehensive research to ensure that these advancements lead to discernible enhancements in clinical outcomes.
In the field of assisted reproductive technologies, the innovative approach of electrophoretic sperm selection presents a promising avenue for improving fertility outcomes. This technique capitalizes on the inherent electrical properties of spermatozoa, leveraging the distinct charge they acquire during maturation to facilitate selective isolation. The progression of this technique from its preliminary stages to the introduction of sophisticated devices like the Felix™ exemplifies the commitment of the field to addressing concerns and refining methodologies. Electrophoretic sperm selection offers a range of advantages, from rapid and non-invasive sperm isolation to augmented sample purity and heightened DNA integrity. Both the CS-10 and Felix™ devices, supplemented by additional pertinent studies, underscore the potential of this technique to revolutionize sperm selection, culminating in elevated success rates for assisted reproduction. However, as is the case with any emergent technology, certain challenges must be meticulously addressed. The nuanced balance between sperm membrane charge and functional attributes, coupled with the imperative for comprehensive validation across diverse semen samples, accentuates the intricacy of this technique. While electrophoretic sperm selection offers considerable potential, ongoing research and meticulous refinement are essential to guarantee its successful integration into clinical practice. In conclusion, the path of electrophoretic sperm selection is a continuous and evolving technology stream, remaining subject to further advancement and exploration. The concerted efforts of both researchers and clinicians have yielded significant progress in addressing concerns and unlocking the potential of this technique. As the field progresses, it is imperative to embrace these advancements while concurrently ensuring rigorous validation and the pursuit of improved fertility outcomes.
Intracytoplasmic morphologically selected sperm injection (IMSI)
Intracytoplasmic Morphologically Selected Sperm Injection (IMSI) stands as a pioneering technique in assisted reproduction, with the primary objective of optimizing the selection of high-quality sperm for fertilization [ 10 ]. This advanced procedure employs high-magnification microscopy, typically around 6000x magnification, to meticulously assess sperm morphology. Through this rigorous assessment of parameters such as nuclear vacuoles, acrosomal integrity, and overall structure characteristics, IMSI seeks to pinpoint sperm that possess the optimal genetic and structural attributes [ 10 , 28 ].
This approach offers a range of potential advantages [ 9 , 11 , 28 ](Table 1 ). A foremost advantage of IMSI lies in its capacity to enhance the selection of morphologically normal sperm. By leveraging higher magnification, even minute abnormalities become detectable abnormalities that might be overlooked at lower magnifications [ 28 ]. This enhanced precision is instrumental in singling out sperm with optimal genetic integrity. Moreover, the meticulous selection process of IMSI could mitigate the likelihood of transmitting genetic abnormalities or DNA damage to the developing embryo, potentially leading to improved pregnancy rates [ 11 , 28 ].
However, despite its potential benefits, IMSI presents certain limitations. The exhaustive examination procedure requires considerable time, which could extend treatment durations and elevate potential patient stress [ 28 ]. Furthermore, while promising, compelling evidence delineating clear and significant improvements in clinical outcomes compared to conventional methods like ICSI remains somewhat scarce [ 12 , 28 ]. Additionally, the requisites for specialized equipment and the expertise essential for high-magnification microscopy could contribute to increased costs and intricate procedures, potentially restricting accessibility for certain patients [ 12 , 28 , 29 ]. Firstly, the acquisition and maintenance of such advanced microscopy systems can significantly increase the overall financial strain associated with assisted reproductive treatments. The initial acquisition costs, in tandem with expenses related to routine maintenance, calibration, and prospective upgrades, amplify the financial implications of IMSI procedures compared to conventional methods. These elevated financial requirements could pose challenges for patients with limited financial means, potentially restricting their access to this advanced technique. Furthermore, the complexities associated with high-magnification microscopy necessitate an advanced degree of technical proficiency and specialized training for embryologists and laboratory personnel [ 29 ]. To attain accurate and consistent results, an in-depth grasp of the equipment is imperative, along with meticulous sample preparation and adapt interpretation of the complex morphological details shown by the high-magnification imagery. As a result, clinics offering IMSI must allocate resources towards training programs and continuous professional development for their staff members. This emphasis on specialized training and expertise adds an additional layer of complexity to the overall procedure, potentially necessitating an extended learning curve for embryologists transitioning to this technique. The combination of increased financial expenditures and heightened technical demands collectively contributes to the procedural complexity of IMSI. Although the prospective advantages of IMSI are substantial, the requisites for specialized apparatus, continual maintenance, and advanced technical expertise might render its adoption more challenging for certain clinics and patients. As the field of assisted reproduction endeavors towards inclusivity and egalitarian access to advanced treatments, devising strategies to surmount these challenges emerges as a critical priority. Efforts to enhance training programs, explore cost-effective equipment alternatives, and foster collaborations among clinics could pave the way toward broader accessibility of IMSI. This would ensure that patients from various socio-economic backgrounds have the opportunity to benefit from this advanced sperm selection technique [ 12 , 28 , 29 ].
The subjective element intrinsic to embryologists’ assessment of sperm morphology during the selection process carries the risk of introducing selection bias and result in variability of outcomes [ 12 , 28 ]. This significant concern emphasizes the necessity for standardized criteria and comprehensive methodologies in sperm selection techniques, such as IMSI. Any deviations can markedly impact the success rates and overall efficiency of assisted reproductive procedures. Efforts toward standardization encompass the establishment of comprehensive guidelines for sperm selection criteria and morphological assessments in IMSI. The primary objective is to reduce observer variability. Ongoing work is carefully creating consistent rules to accurately define the parameters for selecting sperm and to enhance the accuracy of morphological assessment within the IMSI process [ 28 ]. This systematic approach aims to effectively diminish the impact of observer variability, ensuring a consistent and objective methodology for embryologists in the process of sperm selection. By minimizing inherent subjectivity through these guidelines, the overall reliability of IMSI outcomes could be markedly augmented, consequently translating into elevated success rates for assisted reproductive procedures. Furthermore, instituting standardized criteria might facilitate the development of exhaustive training initiatives for embryologists, ensuring their proficiency in accurately identifying and selecting morphologically normal sperm. This pedagogical aspect is crucial in ensuring uniformity and competence across different clinical settings. Furthermore, the adoption of standardized guidelines might foster collaboration within the reproductive medicine community, facilitating the exchange of best practices and continuous refinement of the IMSI technique.
As the path to standardization continues, it is imperative that these efforts remain adaptive to new research findings and technological advancements. By directly confronting the issue of observer variability, the field of assisted reproduction may advance towards elevated precision, ultimately benefiting couples seeking fertility treatments. Further exploration delves into the long-term health and developmental outcomes of embryos derived from IMSI-selected sperm [ 9 , 28 ]. Researchers are actively exploring the combined use of IMSI with other advanced sperm selection techniques to leverage their potential synergistic benefits [ 11 , 12 , 28 ]. Moreover, recent studies have investigated integrating techniques like motile sperm organelle morphology examination (MSOME) with IMSI to address cases of unexplained infertility [ 11 ].
In conclusion, IMSI holds substantial promise within the domain of assisted reproduction [ 10 ]. Through the utilization of high-magnification microscopy and stringent morphological criteria, IMSI offers the potential to elevate the quality of selected sperm, ultimately contributing to improved embryo quality and heightened pregnancy rates [ 9 , 10 , 11 , 28 ]. Nonetheless, the present evidence underscores the imperative for ongoing research, efforts towards standardization, and a holistic exanimation to thoroughly assess the efficacy of the technique and its implications for assisted reproductive practices [ 9 , 11 , 12 , 28 , 29 ].
Advanced sperm quality assessment technology
The continuous advancement of technology has unveiled new frontiers in the field of male fertility assessment, offering more sophisticated methods to evaluate sperm quality for successful assisted reproduction [ 30 ]. While traditional semen analysis has long been the cornerstone of assessing male fertility, the limitations of this approach have driven the development of advanced techniques that provide a deeper understanding of sperm characteristics [ 31 ]. These innovative methods offer the potential to revolutionize how we assess and address male infertility, shedding light on previously unexplored aspects of sperm quality [ 13 ].
Sperm chromatin structure assay (SCSA)
The Sperm Chromatin Structure Assay (SCSA) is a technique that evaluates DNA fragmentation in sperm, a critical factor influencing fertilization and subsequent embryo development. This method engages acridine orange staining in conjunction with flow cytometry to ascertain the susceptibility of sperm DNA to denaturation [ 31 ]. Given the intimate correlation between the integrity of sperm DNA and reproductive success, the SCSA emerges as an indispensable instrument in the evaluation of male fertility [ 32 ].
SCSA provides a comprehensive assessment of DNA fragmentation in sperm, contributing to an enhanced comprehension of prospective reproductive results. By quantifying the degree of DNA denaturation, SCSA offers insights into the sperm’s ability to support successful fertilization and embryo development. However, the intricate nature of SCSA necessitates the use of specialized equipment and expertise, potentially heightening the intricacy and expense associated with assessments. Additionally, although SCSA proffers crucial insights into DNA fragmentation, establishing a direct correlation with clinical outcomes remains inconsistent, underlining the need for additional research [ 31 ].
Zeta potential analysis
Zeta potential is an indicator of the surface charge of sperm cells. This analysis is based on the principle of electrophoresis, which measures the movement of charged particles in an electric field, it offers insights into sperm capacitation and their interaction with the female reproductive tract [ 14 ]. As such, a deeper comprehension of the zeta potential and its role in sperm behavior can be pivotal for predicting fertilization success [ 33 ].
Zeta potential analysis provides a unique perspective on sperm functionality by assessing surface charge dynamics. The evaluation of these dynamics can offer insights into sperm capacitation and its role in successful fertilization. While Zeta potential analysis holds promise, its translation into clinical practice might require overcoming challenges related to standardization and establishing clear reference values. Additionally, the complex interplay of a range of factors affecting zeta potential dynamics in vivo necessitates further research [ 14 ].
The integration of advanced sperm quality assessment methodologies marks a monumental advancement forward in the field of assisted reproduction. This potential renaissance stands poised to reshape the landscape of reproductive medicine. The nexus of technological innovation with the intricacies of reproductive biology may serve as the catalyst for optimized outcomes for couples grappling with infertility challenges [ 13 ]. While the path to progress is undeniably accompanied by inherent challenges, it is the persistent commitment to exhaustive research, rigorous standardization, and seamless clinical implementation that will bridge the gap between these cutting-edge techniques and their transformative potential. This journey, powered by the formidable capabilities of technology, is set to illuminate and enrich the path toward parenthood.
As these advanced methods gain traction, the imperative need for standardized protocols and benchmark reference values becomes evident [ 13 , 30 , 32 , 34 ]. Through their integration into routine clinical practice, these methods hold the potential to substantially enhance the outcomes of assisted reproduction, granting clinicians with insightful tools for judicious treatment decisions [ 33 ]. By delving deeper into various sperm parameters, these methodologies offer a comprehensive evaluation of male fertility potential. Despite the complexities inherent to innovation, ongoing research endeavors and the pursuit of standardization will be the linchpin in realizing the full transformative scope of these advanced techniques [ 13 , 30 , 31 , 32 , 34 ].
In this era of reproductive medicine, the integration of technological advancements with sophisticated medical insight facilitates progress towards enhanced and individualized assisted reproduction outcomes. Navigating this evolving terrain, the potential of advanced sperm quality assessment techniques emerges as a pivotal factor, expanding the horizons of parenthood and offering hope to those pursuing its rewards.
Comparison of conventional and advanced sperm selection methods
Evolution of sperm selection techniques.
Advancements in sperm selection techniques have been profound, catalyzed by the unyielding endeavor to enhance outcomes within ART [ 35 ]. The evolution of sperm selection methodologies can be delineated into three pivotal stages: the establishment of historical foundations, the refinement of conventional techniques through iterative development, and the recent integration of sophisticated approaches. In the initial stages, traditional sperm selection techniques, namely swim-up and DGC, established the foundational protocols that catalyzed further advancements in the field [ 15 , 36 ]. These methodologies, which have been fundamental in ART clinics for many years, function by isolating motile spermatozoa through their intrinsic motility or by employing density gradient centrifugation to segregate spermatozoa based on their specific density. These techniques formed the bedrock for preparing high-quality sperm for fertility procedures. As time progressed, enhancements in these traditional methodologies have become manifest. Decades of empirical research and clinical experience have been instrumental in refining the swim-up and DGC techniques [ 37 ]. They have evolved into reliable tools for the efficient isolation of motile sperm and elimination of contaminants, ultimately enhancing the chances of successful fertilization.
A range of innovative methods for sperm selection has emerged over the past few years and heralded the advent of a new era characterized by advanced technologies [ 16 , 38 , 39 ]. These methods leverage on cutting-edge technologies to target distinct attributes of sperm quality. IMSI employs high-magnification microscopy to select sperm based on morphological criteria, whereas microfluidic sperm sorting utilizes microscale channels and fluid dynamics to augment the precision of selection. Zeta potential-based selection utilizes the electrical charge on sperm surfaces, whereas sperm chromatin dispersion (SCD) measurements specifically target DNA fragmentation assessment. This advanced technology exemplifies the field’s commitment to addressing complex facets of sperm quality through customized solutions.
In summary, the trajectory of sperm selection methodologies illustrates a continual progression from foundational practices to refined conventional techniques, culminating in the introduction of advanced methods. This progression underscores the unwavering dedication to enhancing fertility treatment outcomes and demonstrates the dynamic nature of reproductive medicine. Each stage of development enriches the framework for a more comprehensive and personalized approach to sperm selection, congruent with the paramount objective of optimizing ART success.
Conventional vs. advanced sperm selection techniques: a comparative analysis
There exists a between traditional and innovative methods in the field of sperm selection. This section presents a thorough comparative analysis of these approaches, shedding light on the core differences in their underlying principles, distinct focal points on physical and functional attributes, the crucial role of technology in shaping advanced techniques, the utilization of specialized tools and materials, and the future implications that accompany the shift from conventional to advanced methodologies.
At its core, the distinction between conventional and advanced techniques lies in their foundational principles. Traditional methods, typified by DGC and swim-up approaches, are predicated on sedimentation principles to isolate sperm with desirable physical characteristics [ 16 , 28 , 35 , 36 , 37 ]. In contrast, advanced techniques, as demonstrated by IMSI and Zeta potential-based selection, are grounded in more sophisticated methodologies that delve into the complex interactions of spermatozoa’s functional attributes, such as DNA integrity and gene expression [ 4 , 21 , 23 , 25 , 31 ].
The divergent emphases of these techniques are markedly distinct. Conventional methods focus primarily on physical traits, such as motility and morphology, as indicators of sperm quality [ 16 , 28 , 35 , 36 , 37 ]. In contrast, advanced methods, exemplified by IMSI and Zeta potential-based selection, concentrate on functional attributes as potent determinants of fertilization success [ 29 , 34 ]. This shift represents a transformative progression, acknowledging that the genetic integrity and molecular characteristics of sperm are fundamentally interconnected with their ability to achieve successful fertilization.
Technology serves as a pivotal catalyst in the evolution of advanced sperm selection techniques. Microfluidic platforms, representing the forefront of innovation, utilizing fluid dynamics to meticulously manipulate and segregate sperm based on their attributes [ 9 , 28 ]. Such technological advancements permit the rapid and precise selection of sperm with optimal qualities, thereby revolutionizing the paradigm of sperm selection methodologies. Utilizing specialized tools and materials further underscores the chasm between conventional and advanced approaches. Microfluidic devices, for instance, employ intricate channel designs to facilitate controlled sperm separation, enabling improved DNA integrity and motility [ 9 , 28 ]. Similarly, Zeta potential-based selection leverages unique chamber configurations to exploit sperm’s intrinsic charge, exemplifying the ingenuity inherent in advanced methods [ 34 ].
Technology functions as a pivotal driving force in the evolution of advanced reproductive selection techniques. Microfluidic platforms have risen to prominence as state-of-the-art instruments, utilizing fluid dynamics to meticulously control and categorize spermatozoa according to their distinct characteristics [ 9 , 28 ]. Such technological advancements facilitate the swift and accurate identification of spermatozoa possessing desirable qualities, thereby transforming the field of sperm selection methodologies. The utilization of specialized tools and materials distinctly emphasizes the chasm between conventional and advanced approaches. For example, microfluidic devices utilize complex channel designs to enable precise sperm separation, thereby enhancing DNA integrity and motility [ 9 , 28 ]. Similarly, Zeta potential-based selection leverages specialized chamber configurations to tap into the inherent electrical charge of spermatozoa, showcasing the innovation embedded in advanced methods [ 34 ].
The transition from conventional to advanced methods holds profound future implications. Clinical trials have showcased the potential of these advanced techniques to yield superior outcomes. Zeta potential-based selection has been shown to improve embryo quality and increase the rate of successful pregnancies compared to traditional DGC methods [ 34 ]. Likewise, microfluidic sperm sorting has demonstrated the capability to enhance sperm concentration, motility, and embryo quality in ICSI treatments [ 9 ]. This empirical evidence indicates a paradigm shift, wherein functional attributes are increasingly recognized as paramount in maximizing treatment success.
The comparative analysis of conventional and advanced sperm selection techniques encompasses multiple dimensions (Table 2 ). Spanning from foundational principles to technological advancements, physical attributes, and functional intricacies, this analysis underscores the profound shift in the approach to treating male factor infertility. As advanced methodologies increasingly gain prominence, the synergistic interplay of science and technology ushers in new frontiers, heralding more precise, efficacious, and personalized approaches to enhancing fertility.
The transition from traditional to improved sperm selection approaches constitutes a substantial advancement in the field of ART. Conventional approaches, which rely on sedimentation and density principles, have historically formed cornerstone of sperm selection in ART. These conventional methods predominantly focused on physical characteristics, such as motility and morphology. While these factors are undoubtedly important, they offer only a limited insight of the complicated world of male fertility. However, the advent of advanced techniques marks a fundamental shift in this field. These methods, grounded in the foundations of sophisticated scientific methodologies and cutting-edge technology, rigorously investigate the functional attributes of sperm. These methods take into account factors such as DNA integrity, gene expression, and capacitation potential, acknowledging the critical role these molecular attributes play in the path to successful fertilization. This transition underscores the significance of comprehending sperm at a molecular level, transcending the focus on mere physical characteristics.
The empirical evidence underpinning this shift is compelling. Clinical trials have consistently demonstrated the capability of advanced techniques to surpass the performance of their conventional counterparts. For instance, Zeta potential-based selection has demonstrated notable efficacy, improving embryo quality and increasing the likelihood of successful pregnancies, particularly when compared to traditional DGC methods [ 15 , 34 , 35 ]. Similarly, microfluidic sperm sorting has demonstrated its effectiveness by elevating sperm concentration, motility, and embryo quality in ICSI treatments [ 4 , 56 ]. These findings signify a paradigm shift where functional attributes increasingly assume a central role in optimizing treatment success.
This comparative analysis between conventional and advanced sperm selection techniques encompasses multiple dimensions (Table 2 ). It represents not merely a transition in methodology but signifies the evolution of an entire field. The synergistic interplay of science and technology is forging new frontiers, heralding the advent of more precise, effective, and personalized approaches to enhancing fertility. Advanced methodologies transcend mere techniques; they epitomize a transformative journey toward more successful ART outcomes and actualizing the aspirations of parenthood.
In conclusion, the transition from conventional to advanced sperm selection techniques exemplifies the extraordinary potential of medical science and innovation to transform and expand the realm of possibilities. This advancement offers newfound hope to individuals and couples struggling with infertility challenges. As advanced techniques increasingly gain prominence, the future holds the promise of more tailored, efficient, and effective solutions, ultimately enhancing the probabilities of successful pregnancies and fulfilling the fundamental human aspiration for parenthood [ 9 , 29 , 39 ].
Future directions and implications
The evolution of sperm selection techniques from conventional methods to advanced approaches has revolutionized assisted reproduction, opening avenues for a future replete with possibilities. This section examines the transformative impact of advanced sperm selection methods and delves into the future implications of these developments. A comprehensive table detailing the evolution of sperm selection techniques, their benefits for commercialization in the ART industry, and their congruence with patient preferences is included (Table 3 ). This table provides an overview of the strategic advantages of advanced methods, delineating their role as essential elements of contemporary assisted reproductive technology.
The groundbreaking advancements in sperm selection have heralded the advent of a new era in assisted reproduction. Transitioning from basic motility assessments, the field has evolved to sophisticated functional and genetic evaluations, yielding enhancements in fertilization rates, embryo quality, and pregnancy outcomes [ 39 , 56 ]. As these techniques increasingly gain prominence, there emerges a need to reconcile the advantages they provide with the proven historical success of conventional methods that have withstood the test of time [ 16 , 36 ]. Customization and individualized approaches are increasingly recognized as crucial factors in determining the most appropriate sperm selection method for each specific case. Patient-centric care is now enhanced by the availability of diverse techniques, enabling fertility specialists to customize treatments in accordance with individual needs and preferences [ 15 , 34 ]. The integration of advanced sperm selection methods empowers clinicians to precisely address complex cases, thereby offering patients an increased likelihood of successful outcomes [ 39 , 56 ].
In the realm of assisted reproduction, the significance of ongoing research and development is paramount. The current advancements represent merely a stepping stone towards a future wherein sperm selection outcomes are optimized to an unprecedented level. Scientific inquiry dedicated to refining existing techniques and exploring novel methodologies, as exemplified in reference [ 4 ], will persistently influence landscape of fertility treatments [ 37 , 38 ]. The synergistic interplay of clinical insights and technological innovation hold the promise of elevating the success rate of assisted reproduction.
The incorporation of advanced technologies into assisted reproductive practices engenders a sense of optimism for the future advancements. As these methods grow more accessible and sophisticated, the spectrum of fertility treatments expended. Both patients and clinicians can look forward to more personalized, efficacious, and streamlined solutions, potentially resulting in increased rates of successful pregnancies and the realization of aspirations for parenthood [ 29 , 39 ]. The transition from conventional to advanced sperm selection techniques epitomizes the potential of science and innovation to reshape lives and forge new frontiers in assisted reproduction.
Charting progress: a holistic perspective
The evolution from conventional sperm selection methods to advanced techniques signifies a transformative journey, profoundly impacting the landscape of assisted reproduction. This section conducts a comprehensive analysis of this transformative journey, providing a holistic perspective on its implications and far-reaching impact. The transition from motility-based assessments to methods focused on functional attributes and genetic integrity, as detailed in reference [ 4 ], constitutes a defining characteristic of this transformation. Each advancement in this field has been propelled by the dual goals of achieving higher success rates and improved patient experiences [ 39 , 56 ]. This evolutionary trajectory serves as a testament to the resilience of the field, exemplifying its commitment to pushing boundaries and unlocking novel avenues in fertility treatment.
As advanced sperm selection methods assume a central role, their implications extend well beyond the walls of laboratories and clinics. These techniques have elevated the likelihood of successful conception and fundamentally transformed the dialogue around infertility. These advancements have fostered optimism and enabled couples to consider a wider array of fertility treatments, each specifically tailored to their unique needs and circumstances [ 4 , 25 ]. The far-reaching impact of these advancements extends beyond individual cases, potentially altering societal attitudes toward assisted reproduction. The success stories emerging from advanced techniques, including those studied in reference [ 4 ], possess the capability to inspire, educate, and encourage a broader acceptance of these technologies. As more families experience the joy of parenthood through these methods, the perception of infertility can gradually evolve from a source of stigma to one of resilience and hope [ 25 , 56 ].
In conclusion, the transformative journey from conventional to advanced sperm selection methods epitomizes the union of scientific innovation and the human quest for parenthood. This evolution stands as a testament to the extraordinary ability of medical science to transcend limitations and redefine the realm of possibilities. The impact of these advancements reaches beyond merely improving pregnancy rates; it profoundly affects the lives of individuals and families. For many, the long-held dream of parenthood has been realized through the dedicated efforts of clinicians, researchers, and groundbreaking technologies.
In conclusion, the transformation from traditional to advanced sperm selection techniques exemplifies the integration of scientific innovation with the pursuit of parenthood, signifying a major advancement in ART. Methods such as microfluidic sperm sorting, MACS, and IMSI each uniquely enhance gamete and embryo quality by prioritizing genetic integrity and functional competence. This progression towards more accurate and inclusive gamete selection marks a pivotal change in fertility treatments, aiming for personalized, accessible, and ethically sound options. The effects of these innovations go beyond merely increasing pregnancy rates; they profoundly impact the lives of individuals and families for whom the dream of parenthood is realized through the combined efforts of clinicians, researchers, and cutting-edge technologies. As these techniques advance, they promise to enhance success rates and overcome existing challenges, steering ART toward more equitable and effective solutions. Ultimately, these developments lay the foundation for the future of personalized fertility care, offering promising prospects for optimized outcomes and enhanced patient care, thus transforming societal views on infertility from a condition marked by stigma to one characterized by resilience and hope.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
Assisted Reproductive Technology
Magnetic-Activated Cell Sorting
Intracytoplasmic Morphologically Selected Sperm Injection
polydimethylsiloxane
phosphatidylserine
density gradient centrifugation
Intracytoplasmic Sperm Injection
motile sperm organelle morphology examination
Sperm Chromatin Structure Assay
sperm chromatin dispersion
Schuster TG, Cho B, Keller LM, Takayama S, Smith GD. Isolation of motile spermatozoa from semen samples using microfluidics. Reprod Biomed Online. 2003;7:75–81.
Article PubMed Google Scholar
Mirsanei JS, Sheibak N, Zandieh Z, Mehdizadeh M, Aflatoonian R, Tabatabaei M, et al. Microfluidic chips as a method for sperm selection improve fertilization rate in couples with fertilization failure. Arch Gynecol Obstet. 2022;306:901–10.
Article CAS PubMed Google Scholar
Anbari F, khalili MA, Sultan Ahamed AM, Mangoli E, Nabi A, Dehghanpour F, et al. Microfluidic sperm selection yields higher sperm quality compared to conventional method in ICSI program: a pilot study. Syst Biol Reprod Med. 2021;67:137–43.
Gil Juliá M, Hervas I, Navarro-Gomezlechon A, Mossetti L, Quintana F, Amoros D et al. Semen processing using magnetic-activated cell sorting before ICSI is deemed safe for obstetric and perinatal outcomes: a retrospective multicentre study. Reprod Biomed Online. 2023.
Salehi Novin M, Mehdizadeh A, Artimani T, Bakhtiari M, Mehdizadeh M, Aflatoonian R et al. MACS-DGC sperm preparation method resulted in high-quality sperm, top-quality embryo, and higher blastocyst rate in male factor infertile couples with high DNA fragmented sperm. Hum Fertil. 2023;1–9.
Lee TH, Liu CH, Shih YT, Tsao HM, Huang CC, Chen HH, et al. Magnetic-activated cell sorting for sperm preparation reduces spermatozoa with apoptotic markers and improves the acrosome reaction in couples with unexplained infertility. Hum Reprod. 2010;25:839–46.
Zhang H, Xuan X, Yang S, Li X, Xu C, Gao X. Selection of viable human spermatozoa with low levels of DNA fragmentation from an immotile population using density gradient centrifugation and magnetic-activated cell sorting. Andrologia. 2018;50:1–7.
Article Google Scholar
Fleming SD, Ilad RS, Griffin AMG, Wu Y, Ong KJ, Smith HC, et al. Prospective controlled trial of an electrophoretic method of sperm preparation for assisted reproduction: comparison with density gradient centrifugation. Hum Reprod. 2008;23:2646–51.
Moubasher A, Abdel-Raheem T, Ahmed H, Salem A, Doshi A, Abdel Raheem A. An open prospective study on whether intracytoplasmic morphologically selected sperm injection (IMSI) offers a better outcome than conventional intracytoplasmic sperm injection (ICSI). Cureus. 2021;13:1–13.
Google Scholar
Emirdar V, Acet F. The effect of azoospermia factor microdeletions on intracytoplasmic sperm injection results in azoospermia patients. Pak J Med Sci. 2023;39:672–6.
Article PubMed PubMed Central Google Scholar
Asali A, Miller N, Pasternak Y, Freger V, Belenky M, Berkovitz A. The possibility of integrating motile sperm organelle morphology examination (MSOME) with intracytoplasmic morphologically-selected sperm injection (IMSI) when treating couples with unexplained infertility. PLoS ONE. 2020;15:1–9.
Teixeira DM, Hadyme Miyague A, Barbosa MAP, Navarro PA, Raine-Fenning N, Nastri CO, et al. Regular (ICSI) versus ultra-high magnification (IMSI) sperm selection for assisted reproduction. Cochrane Database of Systematic Reviews. John Wiley and Sons Ltd; 2020. pp. 1–47.
Chatzimeletiou K, Fleva A, Nikolopoulos TT, Markopoulou M, Zervakakou G, Papanikolaou K, et al. Evaluation of sperm DNA fragmentation using two different methods: TUNEL via Fluorescence Microscopy, and Flow Cytometry. Med (Kaunas). 2023;59:1–12.
Zahedi A, Tavalaee M, Deemeh MR, Azadi L, Fazilati M, Nasr-Esfahani MH. Zeta potential vs apoptotic marker: which is more suitable for ICSI sperm selection? J Assist Reprod Genet. 2013;30:1181–6.
Article CAS PubMed PubMed Central Google Scholar
Cabello Y, Belchín P, González-Martínez M, López-Fernández C, Johnston S, Gosálvez J. The efficacy of novel centrifugation-free sperm selection (Io-Lix) on sperm parameters and ICSI reproductive outcomes. Reprod Biomed Online. 2023;46:267–73.
Brahem S, Mehdi M, Elghezal H, Saad A. Semen processing by density gradient centrifugation is useful in selecting sperm with higher double-strand DNA integrity. Andrologia. 2011;43:196–202.
Eravuchira PJ, Mirsky SK, Barnea I, Levi M, Balberg M, Shaked NT. Individual sperm selection by microfluidics integrated with interferometric phase microscopy. Methods. 2018;136:152–9.
Venugopal D, Kasani N, Manjunath Y, Li G, Kaifi JT, Kwon JW. Clog-free high-throughput microfluidic cell isolation with multifunctional microposts. Sci Rep. 2021;11:1–8.
Clark AS, San-Miguel A. A bioinspired, passive microfluidic lobe filtration system. 2021.
Bibi R, Jahan S, Afsar T, Almajwal A, Hammadeh ME, Amor H, et al. Analyzing the Differential Impact of Semen Preparation methods on the outcomes of assisted Reproductive techniques. Biomedicines. 2023;11:1–15.
Degheidy T, Abdelfattah H, Seif A, Albuz FK, Gazi S, Abbas S. Magnetic activated cell sorting: an effective method for reduction of sperm DNA fragmentation in varicocele men prior to assisted reproductive techniques. Andrologia. 2015;47:892–6.
CAS PubMed Google Scholar
Delbes G, Herrero MB, Troeung ET, Chan PTK. The use of complimentary assays to evaluate the enrichment of human sperm quality in asthenoteratozoospermic and teratozoospermic samples processed with Annexin-V magnetic activated cell sorting. Andrology. 2013;1:698–706.
Nadalini M, Tarozzi N, Di Santo M, Borini A. Annexin V magnetic-activated cell sorting versus swim-up for the selection of human sperm in ART: is the new approach better then the traditional one? J Assist Reprod Genet. 2014;31:1045–51.
Mei J, Chen LJ, Zhu XX, Yu W, Gao QQ, Sun HX, et al. Magnetic-activated cell sorting of nonapoptotic spermatozoa with a high DNA fragmentation index improves the live birth rate and decreases transfer cycles of IVF/ICSI. Asian J Androl. 2022;24:367–72.
Villeneuve P, Saez F, Hug E, Chorfa A, Guiton R, Schubert B et al. Spermatozoa isolation with Felix™ outperforms conventional density gradient centrifugation preparation in selecting cells with low DNA damage. Andrology. 2023;1–12.
Simon L, Murphy K, Aston KI, Emery BR, Hotaling JM, Carrell DT. Optimization of microelectrophoresis to select highly negatively charged sperm. J Assist Reprod Genet. 2016;33:679–88.
Ainsworth C, Nixon B, Aitken RJ. Development of a novel electrophoretic system for the isolation of human spermatozoa. Hum Reprod. 2005;20:2261–70.
De Vos A, Van De Velde H, Bocken G, Eylenbosch G, Franceus N, Meersdom G, et al. Does intracytoplasmic morphologically selected sperm injection improve embryo development? A randomized sibling-oocyte study. Hum Reprod. 2013;28:617–26.
Mangoli E, Khalili MA, Talebi AR, Kalantar SM, Montazeri F, Agharahimi A, et al. Association between early embryo morphokinetics plus transcript levels of sperm apoptotic genes and clinical outcomes in IMSI and ICSI cycles of male factor patients. J Assist Reprod Genet. 2020;37:2555–67.
Ghorbani-Sini R, Izadi T, Tavalaee M, Azadi L, Hajian M, Rahimi Zamani M, et al. Comparison of sperm telomere length between two sperm selection procedures: density gradient centrifugation and zeta potential. Int J Fertil Steril. 2020;14:51–6.
CAS PubMed PubMed Central Google Scholar
Evenson DP, Wixon R. Clinical aspects of sperm DNA fragmentation detection and male infertility. Theriogenology. Elsevier Inc.; 2006. pp. 979–91.
Miller D, Pavitt S, Sharma V, Forbes G, Hooper R, Bhattacharya S, et al. Physiological, hyaluronan-selected intracytoplasmic sperm injection for infertility treatment (HABSelect): a parallel, two-group, randomised trial. Lancet. 2019;393:416–22.
Liu KS, Mao XD, Pan F, Chen YJ, An R. Correlation analysis of sperm DNA fragmentation index with semen parameters and the effect of sperm DFI on outcomes of ART. Sci Rep. 2023;13.
Karimi N, Kouchesfahani HM, Nasr-Esfahani MH, Tavalaee M, Shahverdi A, Choobineh H. DGC/Zeta as a New Strategy to improve clinical outcome in male factor infertility patients following intracytoplasmic sperm injection: a randomized, single-blind, clinical trial. Cell J. 2020;22:55–9.
PubMed Google Scholar
Brandeis VT, Manuel MT. CLINICAL ASSISTED REPRODUCTION effects of four methods of sperm Preparation on the motile concentration, morphology, and Acrosome Status of recovered sperm from normal semen samples 1. J Assist Reprod Genet. 1993;10:409–16.
Wang M, Sun J, Wang L, Gao X, Lu X, Wu Z, et al. Assessment of density gradient centrifugation (DGC) and sperm chromatin dispersion (SCD) measurements in couples with male factor infertility undergoing ICSI. J Assist Reprod Genet. 2014;31:1655–63.
Tavalaee M, Deemeh MR, Arbabian M, Nasr-Esfahani MH. Density gradient centrifugation before or after magnetic-activated cell sorting: which technique is more useful for clinical sperm selection? J Assist Reprod Genet. 2012;29:31–8.
Heidari M, Lakpour N, Darbandi M, Darbani S, Shani S, Goharbakhsh L, et al. Upstream or swim up processing technique: which one is more effective to select human sperm with high chromatin integrity. Int J Reprod BioMed. 2018;16:463–8.
Muratori M, Tarozzi N, Carpentiero F, Danti S, Perrone FM, Cambi M, et al. Sperm selection with density gradient centrifugation and swim up: effect on DNA fragmentation in viable spermatozoa. Sci Rep. 2019;9:1–12.
Article CAS Google Scholar
Jahangiri AR, Ziarati N, Dadkhah E, Bucak MN, Rahimizadeh P, Shahverdi A, et al. Microfluidics: the future of sperm selection in assisted reproduction. Andrology. John Wiley and Sons Inc; 2023.
Nosrati R, Graham PJ, Zhang B, Riordon J, Lagunov A, Hannam TG et al. Microfluidics for sperm analysis and selection. Nat Rev Urol Nat Publishing Group; 2017. p. 707–30.
Vaughan DA, Sakkas D, Gardner DK. Sperm selection methods in the 21st century. Biol Reprod. Oxford University Press; 2019. pp. 1076–82.
Ozcan P, Takmaz T, Yazici MGK, Alagoz OA, Yesiladali M, Sevket O, et al. Does the use of microfluidic sperm sorting for the sperm selection improve in vitro fertilization success rates in male factor infertility? J Obstet Gynecol Res. 2021;47:382–8.
Knowlton SM, Sadasivam M, Tasoglu S. Microfluidics for sperm research. Trends Biotechnol. Elsevier Ltd; 2015. pp. 221–9.
Pacheco A, Blanco A, Bronet F, Cruz M, García-Fernández J, García-Velasco JA. Magnetic-activated cell sorting (macs): a useful sperm-selection technique in cases of high levels of sperm dna fragmentation. J Clin Med. 2020;9:1–9.
Garrido N, Gil Juliá M. The use of non-apoptotic sperm selected by magnetic activated cell sorting (MACS) to Enhance Reproductive outcomes: what the evidence says. Biology (Basel). 2024;13:30.
Ziarati N, Tavalaee M, Bahadorani M, Nasr Esfahani MH. Clinical outcomes of magnetic activated sperm sorting in infertile men candidate for ICSI. Hum Fertil. 2019;22:118–25.
Mangoli E, Khalili MA. The beneficial role of intra cytoplasmic morphologically selected sperm injection (IMSI) in assisted Reproduction. J Reprod Infertil. 2020.
Duran-Retamal M, Morris G, Achilli C, Gaunt M, Theodorou E, Saab W, et al. Live birth and miscarriage rate following intracytoplasmic morphologically selected sperm injection vs intracytoplasmic sperm injection: an updated systematic review and meta-analysis. Acta Obstet Gynecol Scand. Wiley-Blackwell;; 2020. pp. 24–33.
Guy Cassuto N, Bouret D, Hatem G, Larue L, Lédée N, Ruoso L et al. Using high magnification to select sperm: a large prospective cohort study comparing ICSI and IMSI. Clin Obstet Gynecol Reprod Med. 2020;6.
Dieamant F, Petersen CG, Vagnini LD, Renzi A, Petersen B, Massaro F, et al. Impact of intracytoplasmic morphologically selected sperm injection (Imsi) on birth defects: a systematic review and meta-analysis. J Bras Reprod Assist. 2021;25:466–72.
Ionov M, Gontarek W, Bryszewska M. Zeta potential technique for analyzing semen quality. MethodsX. 2020;7.
Marzano G, Chiriacò MS, Primiceri E, Dell’Aquila ME, Ramalho-Santos J, Zara V, et al. Sperm selection in assisted reproduction: a review of established methods and cutting-edge possibilities. Biotechnol Adv. Elsevier Inc.; 2020.
Ribas-Maynou J, Barranco I, Sorolla-Segura M, Llavanera M, Delgado-Bermúdez A, Yeste M. Advanced sperm selection strategies as a treatment for infertile couples: a systematic review. Int J Mol Sci MDPI; 2022.
Cakar Z, Cetinkaya B, Aras D, Koca B, Ozkavukcu S, Kaplanoglu İ, et al. Does combining magnetic-activated cell sorting with density gradient or swim-up improve sperm selection? J Assist Reprod Genet. 2016;33:1059–65.
Göde F, Sami Gürbüz A, Tamer B, Pala İ, Isik AZ. The effects of Microfluidic sperm sorting, Density Gradient and Swim-up Methods on Semen Oxidation Reduction Potential.
Sheikhi A, Jalali M, Gholamian M, Jafarzadeh A, Jannati S, Mousavifar N. Elimination of apoptotic spermatozoa by magnetic-activated cell sorting improves the fertilization rate of couples treated with ICSI procedure. Andrology. 2013;1:845–9.
Said TM, Grunewald S, Paasch U, Rasch M, Agarwal A, Glander HJ. Effects of magnetic-activated cell sorting on sperm motility and cryosurvival rates. Fertil Steril. 2005;83:1442–6.
Martínez MG, Sánchez-Martín P, Dorado-Silva M, Fernández JL, Girones E, Johnston SD, et al. Magnetic-activated cell sorting is not completely effective at reducing sperm DNA fragmentation. J Assist Reprod Genet. 2018;35:2215–21.
Download references
Acknowledgements
Not applicable.
This work was supported by National Institutes of Health Grants (No. 1R21CA260989-01A1); Science and Technology Project of Guangdong Province (No. 2015B020226001); Mitacs Elevate Fellowship (No. IT34873).
Author information
Xiang Zhang and Shuen Chao are co-first author.
Authors and Affiliations
College of Animal Science and Technology, China Agricultural University, Beijing, 100193, China
Xiang Zhang
Center for Engineering in Medicine and Surgery, Massachusetts General Hospital, Harvard Medical School, Charlestown, MA, 02129, USA
Shuen Chao, Ningxin Ye & Dongfang Ouyang
Department of Mechanical and Industrial Engineering, University of Toronto, Toronto, M5S 3G8, Canada
Dongfang Ouyang
Department of Biology, University of Ottawa, Ottawa, ON, K1N 6N5, Canada
You can also search for this author in PubMed Google Scholar
Contributions
X.Z. and D.O. wrote the main manuscript text and S.C. prepared Tables 1, 2 and 3. N.Y prepared Tables 4, 5 and 6. All authors reviewed the manuscript.
Corresponding author
Correspondence to Dongfang Ouyang .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Zhang, X., Chao, S., Ye, N. et al. Emerging trends in sperm selection: enhancing success rates in assisted reproduction. Reprod Biol Endocrinol 22 , 67 (2024). https://doi.org/10.1186/s12958-024-01239-1
Download citation
Received : 11 February 2024
Accepted : 01 June 2024
Published : 14 June 2024
DOI : https://doi.org/10.1186/s12958-024-01239-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Assisted Reproductive Technology (ART)
- Sperm selection techniques
- Intracytoplasmic sperm injection (ICSI)
- Sperm Quality Assessment
Reproductive Biology and Endocrinology
ISSN: 1477-7827
- General enquiries: [email protected]
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Alberts B, Johnson A, Lewis J, et al. Molecular Biology of the Cell. 4th edition. New York: Garland Science; 2002.
By agreement with the publisher, this book is accessible by the search feature, but cannot be browsed.

Molecular Biology of the Cell. 4th edition.
In most species, there are just two types of gamete , and they are radically different. The egg is among the largest cells in an organism, while the sperm ( spermatozoon , plural spermatozoa ) is often the smallest. The egg and the sperm are optimized in opposite ways for the propagation of the genes they carry. The egg is nonmotile and aids the survival of the maternal genes by providing large stocks of raw materials for growth and development , together with an effective protective wrapping. The sperm, by contrast, is optimized to propagate the paternal genes by exploiting this maternal investment: it is usually highly motile and streamlined for speed and efficiency in the task of fertilization . Competition between sperm is fierce, and the vast majority fail in their mission: of the billions of sperm released during the reproductive life of a human male, only a few ever manage to fertilize an egg.
- Sperm Are Highly Adapted for Delivering Their DNA to an Egg
Typical sperm are “stripped-down” cells, equipped with a strong flagellum to propel them through an aqueous medium but unencumbered by cytoplasmic organelles such as ribosomes, endoplasmic reticulum , or Golgi apparatus, which are unnecessary for the task of delivering the DNA to the egg . Sperm, however, contain many mitochondria strategically placed where they can most efficiently power the flagellum. Sperm usually consist of two morphologically and functionally distinct regions enclosed by a single plasma membrane : the tail, which propels the sperm to the egg and helps it to burrow through the egg coat, and the head, which contains a condensed haploid nucleus ( Figure 20-25 ). The DNA in the nucleus is extremely tightly packed, so that its volume is minimized for transport, and transcription is shut down. The chromosomes of many sperm have dispensed with the histones of somatic cells and are packed instead with simple, highly positively charged proteins called protamines.
Figure 20-25
A human sperm. It is shown in longitudinal section.
In the head of most animal sperm, closely apposed to the anterior end of the nuclear envelope , is a specialized secretory vesicle called the acrosomal vesicle (see Figure 20-25 ). This vesicle contains hydrolytic enzymes that may help the sperm to penetrate the egg 's outer coat. When a sperm contacts an egg, the contents of the vesicle are released by exocytosis in the so-called acrosome reaction ; in some sperm, this reaction also exposes or releases specific proteins that help bind the sperm tightly to the egg coat.
The motile tail of a sperm is a long flagellum, whose central axoneme emanates from a basal body situated just posterior to the nucleus . As described in Chapter 16, the axoneme consists of two central singlet microtubules surrounded by nine evenly spaced microtubule doublets. The flagellum of some sperm (including those of mammals) differs from other flagella in that the usual 9 + 2 pattern of the axoneme is further surrounded by nine outer dense fibers ( Figure 20-26 ). These dense fibers are stiff and noncontractile, and it is not known what role they have in the active bending of the flagellum, which is caused by the sliding of adjacent microtubule doublets past one another. Flagellar movement is driven by dynein motor proteins, which use the energy of ATP hydrolysis to slide the microtubules, as discussed in Chapter 16. The ATP is generated by highly specialized mitochondria in the anterior part of the sperm tail (called the midpiece), where the ATP is needed (see Figures 20-25 and 20-26 ).
Figure 20-26
Drawing of the midpiece of a mammalian sperm as seen in cross section in an electron microscope. The core of the flagellum is composed of an axoneme surrounded by nine dense fibers. The axoneme consists of two singlet microtubules surrounded by nine microtubule (more...)
- Sperm Are Produced Continuously in Most Mammals
In mammals, there are major differences in the way in which eggs are produced ( oogenesis ) and the way in which sperm are produced ( spermatogenesis ). In human females, for example, oogonia proliferate only in the fetus, enter meiosis before birth, and become arrested as oocytes in the first meiotic prophase , in which state they may remain for up to 50 years. Individual oocytes mature from this strictly limited stock and are ovulated at intervals, generally one at a time, beginning at puberty. In human males, by contrast, meiosis and spermatogenesis do not begin in the testes until puberty and then go on continuously in the epithelial lining of very long, tightly coiled tubes, called seminiferous tubules. Immature germ cells, called spermatogonia (singular, spermatogonium), are located around the outer edge of these tubes next to the basal lamina, where they proliferate continuously by mitosis . Some of the daughter cells stop proliferating and differentiate into primary spermatocytes. These cells enter the first meiotic prophase, in which their paired homologous chromosomes participate in crossing-over , and then proceed with division I of meiosis to produce two secondary spermatocytes, each containing 22 duplicated autosomal chromosomes and either a duplicated X or a duplicated Y chromosome . The two secondary spermatocytes derived from each primary spermatocyte proceed through meiotic division II to produce four spermatids, each with a haploid number of single chromosomes. These haploid spermatids then undergo morphological differentiation into sperm ( Figure 20-27 ), which escape into the lumen of the seminiferous tubule ( Figure 20-28 ). The sperm subsequently pass into the epididymis, a coiled tube overlying the testis, where they undergo further maturation and are stored.
Figure 20-27
The stages of spermatogenesis. Spermatogonia develop from primordial germ cells that migrate into the testis early in embryogenesis. When the animal becomes sexually mature, the spermatogonia begin to proliferate rapidly, generating some progeny that (more...)
Figure 20-28
Highly simplified drawing of a cross section of a seminiferous tubule in a mammalian testis. (A) All of the stages of spermatogenesis shown take place while the developing gametes are in intimate association with Sertoli cells. These large cells extend (more...)
An intriguing feature of spermatogenesis is that the developing male germ cells fail to complete cytoplasmic division ( cytokinesis ) during mitosis and meiosis . Consequently, large clones of differentiating daughter cells that have descended from one maturing spermatogonium remain connected by cytoplasmic bridges, forming a syncytium ( Figure 20-29 ). The cytoplasmic bridges persist until the very end of sperm differentiation , when individual sperm are released into the tubule lumen . This accounts for the observation that mature sperm arise synchronously in any given area of a seminiferous tubule. But what is the function of the syncytial arrangement?
Figure 20-29
Cytoplasmic bridges in developing sperm cells and their precursors. The progeny of a single maturing spermatogonium remain connected to one another by cytoplasmic bridges throughout their differentiation into mature sperm. For the sake of simplicity, (more...)
Unlike oocytes, sperm undergo most of their differentiation after their nuclei have completed meiosis to become haploid . The presence of cytoplasmic bridges between them, however, means that each developing haploid sperm shares a common cytoplasm with its neighbors. In this way, it can be supplied with all the products of a complete diploid genome . Developing sperm that carry a Y chromosome , for example, can be supplied with essential proteins encoded by genes on the X chromosome. Thus, the diploid genome directs sperm differentiation just as it directs egg differentiation.
Some of the genes that regulate spermatogenesis have been conserved in evolution from flies to humans. The DAZ gene , for example, which encodes an RNA -binding protein and is located on the Y chromosome , is deleted in many infertile men, many of whom cannot make sperm. Two Drosophila genes that are homologous to DAZ are essential for spermatogenesis in the fly. RNA-binding proteins are especially important in spermatogenesis, because many of the genes expressed in the sperm lineage are regulated at the level of RNA translation.
A sperm is usually a small, compact cell, highly specialized for the task of fertilizing an egg . Whereas in human females the total pool of oocytes is produced before birth, in males new germ cells enter meiosis continually from the time of sexual maturation, with each diploid primary spermatocyte giving rise to four haploid mature sperm. The process of sperm differentiation occurs after meiosis is complete, requiring five weeks in humans. Because the maturing spermatogonia and spermatocytes fail to complete cytokinesis , however, the progeny of a single spermatogonium develop as a large syncytium . Sperm differentiation is therefore directed by the products from both parental chromosomes, even though each nucleus is haploid.
- Cite this Page Alberts B, Johnson A, Lewis J, et al. Molecular Biology of the Cell. 4th edition. New York: Garland Science; 2002. Sperm.
- Disable Glossary Links
In this Page
Related items in bookshelf.
- All Textbooks
Recent Activity
- Sperm - Molecular Biology of the Cell Sperm - Molecular Biology of the Cell
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers
Route of the sperm from formation to expulsion
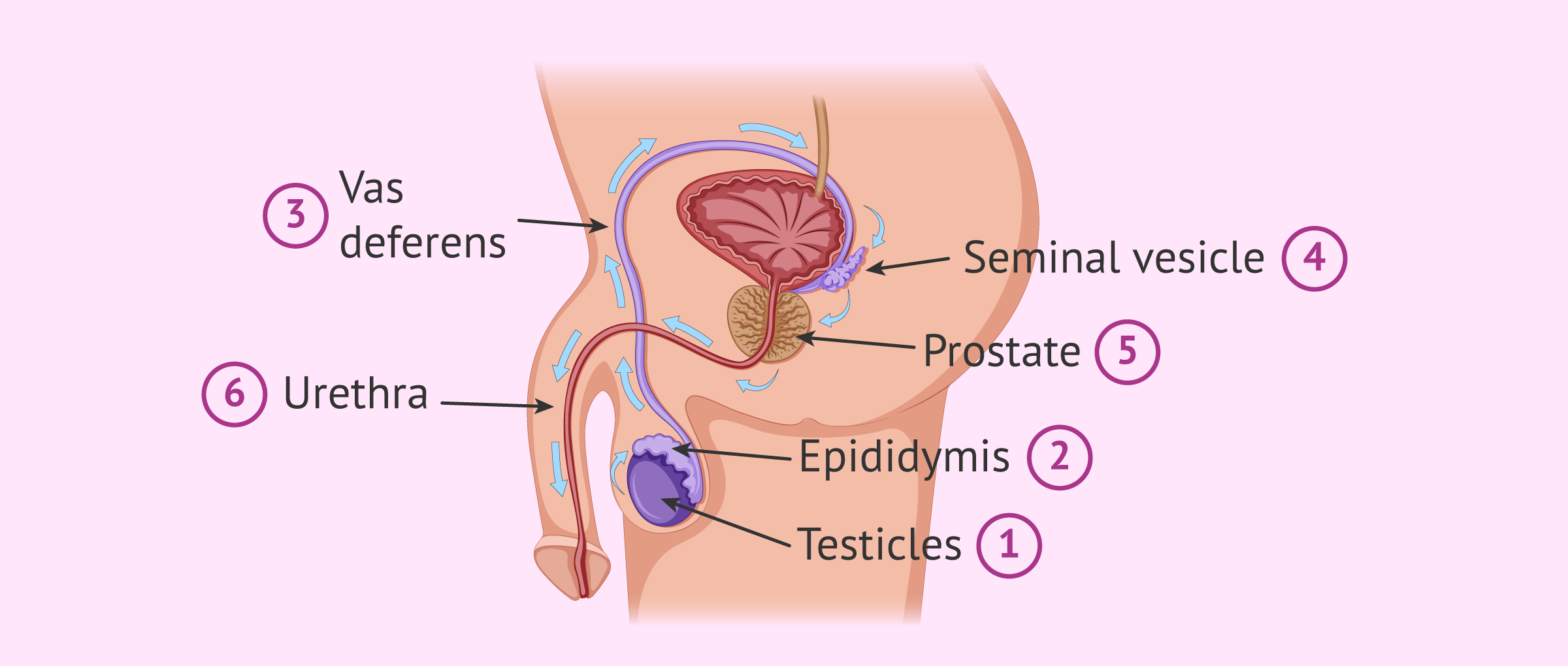
Sperm are produces in the seminiferous tubules of the testis, in a process known as spermatogenesis or sperm production .
To be able to leave the testis and come out in the form of semen, spermatozoa go through the seminiferous tubules of the testis to the epididymis in the first place. Then, they travel inside the vasa deferentia and the urethra. It is at this point when they will be ejected.
During their path inside the male reproductive system before ejaculation, they have to cover themselves with fluids secreted by the seminal vesicle and the prostate, which purpose is to nourish and protect the sperm once inside the woman's body.
Leave a Reply
Privacy Overview

IMAGES
VIDEO
COMMENTS
The journey through the female reproductive system. In the process of ejaculation, sperm cells leave the man and enter the vagina. This is where the sperm cells begin the second part of their journey to fertilization. During this second part of the journey the sperm again encounter an large number of obstacles.
Sperm are haploid; they contain one set of 23 chromosomes. They are created by the cellular division process known as meiosis, which creates 4 sperm from a single germ cell. They're also very small, only about 50μm long. Sperm are ejaculated in semen, a basic fluid with a pH of about 7.4. The sperm's target is the egg.
A sperm cell or spermatozoon is a gamete (sex cell) produced in the male reproductive tract. It is a motile cell with a single aim - to fertilize a female egg. Each sperm cell contains the entire genome of the male that produces it. In combination with the female genome contained within the egg, a zygote is formed - a single totipotent stem ...
To accomplish the journey to the egg, sperm are equipped to overcome various obstacles that lie ahead, such as navigating the uterotubal junction (UTJ) and penetrating the egg extracellular matrices. ... The enzymatic hypothesis for ZP penetration posits that proteolytic cleavage of ZP proteins by sperm cell-surface proteases clears a path ...
Fusion in advanced animals is usually followed by penetration of the egg by a single spermatozoon. The result of fertilization is a cell ( zygote) capable of undergoing cell division to form a new individual. journey of a fertilized egg. The journey of a fertilized egg in a woman. In mammals, eggs are released by the ovaries.
Diagram of the journey of sperm to egg. 4. This diagram shows the entire process of fertilization inside the woman's body. The ultimate goal is to meet the egg in the Fallopian tube. Read the full article on: How sperm meets egg: a journey from production to fertilization ( 43).
spermatogenesis, the origin and development of the sperm cells within the male reproductive organs, the testes.The testes are composed of numerous thin tightly coiled tubules known as the seminiferous tubules; the sperm cells are produced within the walls of the tubules. Within the walls of the tubules, also, are many randomly scattered cells, called Sertoli cells, that function to support and ...
Video summary. This short film uses CGI footage to help give a visual description of the process of human fertilisation, following the journey of both sperm and egg.
The human sperm cell is haploid so that its 23 chromosomes can join the 23 chromosomes of the female egg to form a diploid cell. During fertilization, the sperm provides the following three essential parts to the oocyte: ... This energy is used for the journey through the female cervix, uterus, and uterine tubes. ...
Fertilization: Sperm Penetrates Egg. 5 /9. It takes about 24 hours for a sperm cell to fertilize an egg. When the sperm penetrates the egg, the surface of the egg changes so that no other sperm ...
During this journey through your fallopian tubes, an egg can be fertilized by sperm. Sperm production begins in the testicles of men or people assigned male at birth (AMAB). During ejaculation, millions of sperm cells are set free with the sole purpose of finding an egg to fertilize. When you have unprotected sex, sperm cells swim up through ...
Fertilization is a complex multi-step process that is complete in 24 hours. The sperm from a male meets an ovum from a female and forms a zygote; this is the point in which pregnancy begins and leads to a 280-day journey for a female. There are two ways to track this process, and they differ by the day counting begins. There are the post-ovulation age and the gestational age, calculated by ...
Sperm cells have intrigued biologists since they were first observed nearly 350 years ago by Antonie van Leeuwenhoek and Johan Ham. The discovery of these 'animalcules' (i.e., spermatozoa) launched the field of sperm biology, and the subsequent three and half centuries of inquiry into this small, highly specialized cell has revolutionized our understanding of reproduction and fertilization [].
The journey begins in the testes, where immature sperm cells undergo a process called spermatogenesis. Within special compartments called seminiferous tubules, these cells mature and develop into fully functional spermatozoa. It takes approximately 74 days for this transformation to occur, ensuring a steady supply of healthy and potent sperm.
The sperm cell is a highly polarized and differentiated cell, whose main goal is to reach the oocyte and participate in fertilization . To do so, it must embark on a long journey, and success in this endeavor relies on structurally and functionally sound cells. Despite this common task, sperm cells from different species vary in structure and ...
Sperm ( pl.: sperm or sperms) is the male reproductive cell, or gamete, in anisogamous forms of sexual reproduction (forms in which there is a larger, female reproductive cell and a smaller, male one). Animals produce motile sperm with a tail known as a flagellum, which are known as spermatozoa, while some red algae and fungi produce non-motile ...
Gametes are the male and female sex cells: ova close ovum The egg cell. Plural: ova. are female gametes; sperm are male gametes; ... Describe the journey of a sperm from testis to ovum.
The journey of sperm is an incredible tale of resilience, strategy, and determination. From their origin within the testes to their ultimate goal of fertilization, sperm embark on a treacherous adventure full of challenges and obstacles. ... This mighty hormone orchestrates the construction of new sperm cells while keeping tabs on their quality ...
First, the zona pellucida contains sperm receptors that are specific for human sperm. Second, once penetrated by the sperm, the membrane becomes impermeable to penetration by other sperm. Following penetration, a series of events set the stage for the first cell division. The single-cell embryo is called a zygote.
More than a single sperm cell is required to degrade the ZP. Nonetheless, in the end just one of them will be the "winner", that is, the one who fertilizes the egg. ... Masaru Okabe. Fertilization: a sperm's journey to and interaction with the oocyte. J Clin Invest. 2010 Apr;120(4):984-94. doi: 10.1172/JCI41585. Epub 2010 Apr 1 .
0. The path of the spermatozoa to reach the egg is not a simple one. This path is divided into a phase in the male reproductive system and another in the female reproductive system. In the case of the male, the sperm travel from the testicle to the urethra. When vaginal penetration occurs, the sperm continue their journey to the fallopian tubes.
The Pathway to Fertilization: Understanding the Journey of Sperm . Fertilization is the magical process that brings about new life. And when we talk about fertilization, the first thing that comes to mind is sperm. Sperm cells are like tiny warriors that embark on a perilous journey in search of the ovum.
Researchers have unveiled the hidden intricacies of how sperm go from passive bystanders to dynamic swimmers. This transformation is a pivotal step in the journey to fertilization, and it hinges ...
The remarkable journey that successful sperm take to reach an oocyte is long and tortuous, and includes movement through viscous fluid, avoiding dead ends and hostile immune cells. The privileged collection of sperm that complete this journey must pass selection steps in the vagina, cervix, uterus, utero-tubal junction, and oviduct.
History of Biology; A Short History of Cell Biology. Our journey to understanding that single cells are the fundamental units of life traces back to groundbreaking scientific milestones, such as the invention of the microscope, which revealed individual cells, and advancements like the discovery of fluorescent proteins and electron microscopes that have enriched our insights into the intricate ...
In the film - sold by Charades and debuting an exclusive clip with Variety - two teenagers have sex for the very first time, unaware the sperm cells are readying for their perilous journey ...
This comprehensive review explores the evolving landscape of sperm selection techniques within the realm of Assisted Reproductive Technology (ART). Our analysis delves into a range of methods from traditional approaches like density gradient centrifugation to advanced techniques such as Magnetic-Activated Cell Sorting (MACS) and Intracytoplasmic Morphologically Selected Sperm Injection (IMSI).
In most species, there are just two types of gamete, and they are radically different. The egg is among the largest cells in an organism, while the sperm (spermatozoon, plural spermatozoa) is often the smallest. The egg and the sperm are optimized in opposite ways for the propagation of the genes they carry. The egg is nonmotile and aids the survival of the maternal genes by providing large ...
Route of the sperm from formation to expulsion. 12. Sperm are produces in the seminiferous tubules of the testis, in a process known as spermatogenesis or sperm production. To be able to leave the testis and come out in the form of semen, spermatozoa go through the seminiferous tubules of the testis to the epididymis in the first place.