Clinical Researcher

Clinical Study Reports 101: Tips and Tricks for the Novice
Clinical Researcher September 15, 2020

Clinical Researcher—September 2020 (Volume 34, Issue 8)
PEER REVIEWED
Sheryl Stewart, MCR, CCRP
The tenets of Good Clinical Practice (GCP), promulgated by the International Council for Harmonization (ICH), require that investigator-initiated trials (IITs), especially those involving an Investigational New Drug application to the U.S. Food and Drug Administration (FDA), have the principal investigator (PI), the institution, and the study team assume roles of both the sponsor (ICH GCP E6(R2), Section 5) and of the PI (ICH GCP E6(R2), Section 4).{1} If you are part of an IIT team, whether you are the investigator, a clinical research coordinator, or someone working in any of the many other important roles within the team, you may be tasked with authoring a clinical study report (CSR) at one time or another within the course of the study. At the very least, you may be asked to contribute to, or provide peer review of the document before it is submitted for its intended purpose.
The purpose of this review is to provide a framework for study team members, whether it’s for a large team that includes regulatory and administrative support or for smaller teams with only one or two members, for writing and organizing the CSR.
First, is important to understand the definition, requirements, and potential uses of a CSR. The report is a comprehensive look at all the data produced in a clinical study, presented in text, tables, and figure formats. It will often include discussions and conclusions that provide context to the findings regarding the drug, device, biological product, surgical method, counseling practice, or any other type of therapeutic product or practice under study and where it may contribute to an improvement on the state of the art for treating or preventing a particular health condition.
If a study has prespecified endpoints or parameters, the CSR will report the current outcomes and statistical parameters for these endpoints. Key messages will be referred to and highlighted throughout. Key messages are important study findings that support the prespecified endpoints, supply proof of the justification of clinical benefit, or differentiate the study product from others in the therapeutic space.
Most likely you already appreciate the ethical responsibility a clinical study team has to clinical study data transparency, which for that reason alone would make the production of some sort of CSR necessary. Indeed, the preparation and representation of study progress is prescribed in the aforementioned ICH GCP E6(R2) guideline,{1} which states that study sponsors should ensure that clinical trial reports are prepared and provided to regulatory agencies as they are required.
Further, the guideline recommends study sponsors to rely on a subsequent guideline on Structure and Content of Clinical Study Reports (ICH E3).{2} Lastly, adhering to this ethical responsibility and following GCP have become mandated both in the U.S. and in Europe, where study data are expected to be recorded on ClinicalTrials.gov and the EudraCT database, respectively, for the sake of transparency and in support of further scientific inquiry, thus making the organization and preparation of study data in a prespecified format necessary.{3,4}
There are a few different uses for a CSR, though primarily it is utilized either to summarize the data and outcomes at the end of the study, or for marketing authorization. Those two purposes are specifically outlined in ICH E3 and ICH E6.{1,2} However, a CSR may also be written for third-party payer reimbursement purposes, providing details in support of clinical benefit. Because in most cases CSRs will ultimately have a regulatory reviewer, authoring a report that is consistent in formatting and content with what is expected will hopefully not only enable a smooth review, but also will facilitate proper data cleaning, presentation, and timeliness that make the document fit for purpose.
ICH E3 offers a CSR template to guide you in terms of providing the proper data and content in a specified order and format. This guideline can be found either on the ICH website or the FDA website.{2,5}
It is important to note that there are no requirements to follow the template precisely. Not every section is appropriate for every study, and because the overarching purpose of a CSR is to provide proper representation of the study data and any key messages you want to report, flexibility is allowed and encouraged in order to meet those important goals. However, for anyone new to the process of crafting a CSR, this template is a helpful starting point.
Transcelerate Biopharma, a nonprofit organization involved in researching means to increase efficiency and innovation in the pharmaceutical research sciences, also has interpreted the ICH template and has produced a useful tool to improve this reporting.{6} If the instruction and guidance in the ICH or Transcelerate templates do not meet your needs, or you have further questions as to how to properly represent the study data, the CORE reference manual (Clarity and Openness in Reporting E3-based) is another resource. It was produced in 2016 in response to regulatory changes for public disclosure of clinical study data, and can provide direction and interpretation of the ICH E3 template.{7}
For the novice author of a CSR, however, the ICH E3 template, coupled with the Transcelerate template, should provide a strong starting point for the project planning of the report, as well as the document formatting.
Sidebar: Tips and Tricks for Getting Started
Determining Stakeholders
Once you’ve reviewed the template and created a draft outline of the project, determine the key stakeholders with whom you’ll need to partner to complete this project. Likely you will need input from your clinical study management team, teammates responsible for data entering and cleaning, a biostatistician, any teammate or organization member able to perform literature reviews, those staff qualified to compose patient or adverse event narratives, and those team members who can help determine key messaging in this report. Lastly you will want to determine the group of key stakeholders who will be your final review team for the document—those who will help you finalize the document prior to submission.
Sidebar: Tips and Tricks for Stakeholder and Project Management
Determining Timelines
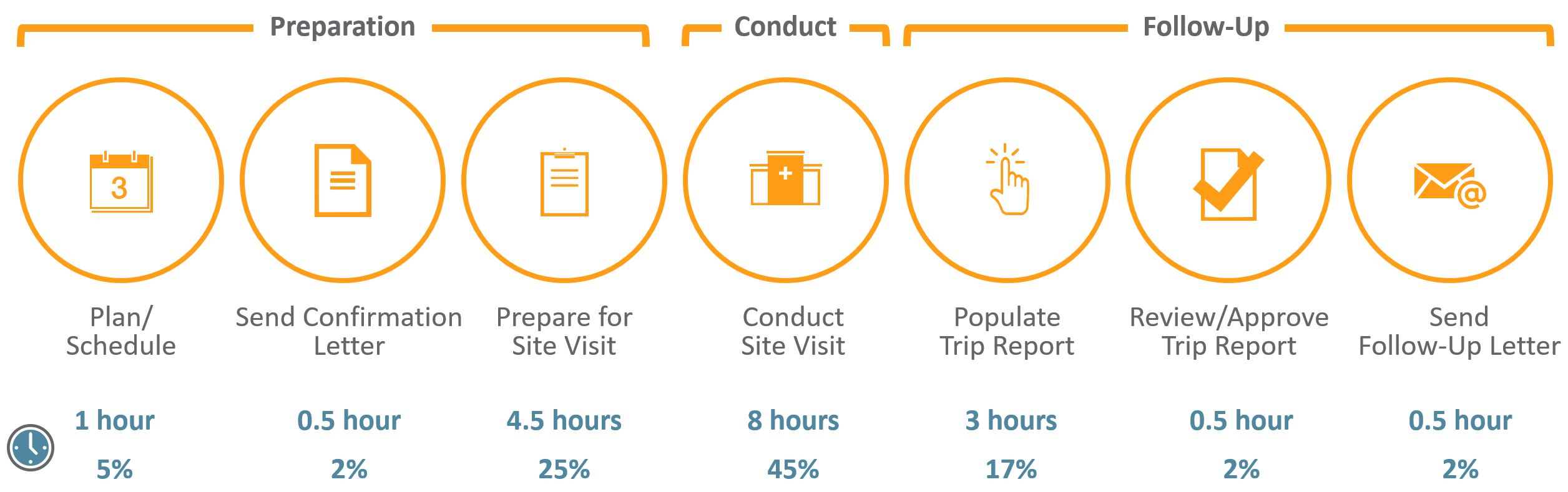
Once you have determined your key stakeholders, you will want to determine timelines to ensure steady progress continues to be made on the document. If you’ve chosen to utilize a scope document, you’ll want to include these timelines in it, so the entire team is aware of the project process, the timing requirements, and each gating item (key gating items are summarized in Figure 1).
Figure 1: Preparing, Writing, and Review of the Clinical Study Report—Key Gating Items
Time management is paramount for clinical trial submissions to regulatory authorities. Attendees at medical writing conferences over the course of a five-year period (2008 to 2013, n=78) were surveyed to determine to how long each step of the CSR process can typically require.{8}
To complete a “moderately complex” CSR for a Phase III study with 200 to 400 participants, the surveyed medical writers responded with a mean answer of 16.9 days from the receipt of the final tables, listings, and figures (TLFs) to delivery of the first draft of the CSR. They estimated a mean of 25.7 days from the first draft to the final draft routed for review. The time from database lock to completion was reported to be on average 83 days.
While there was a wide range for the timelines reported, these data provide the novice CSR author a basic reference point for how long the individual processes can expect to take with experienced medical writers. Fortunately, while TLFs are being crafted, multiple other “Writing and Document Review” tasks from Table 1 can be performed simultaneously.
At Last…the Writing!
Typically, the flow of your CSR will progress under six primary headings or sections, not unlike those used in a research manuscript. On the front end, even before the background and introduction, the document will include a title page, synopsis, table of contents, list of abbreviations, ethics statements, and details on the study’s administrative structure. The primary sections to come after that are highlighted in Figure 2 and summarized in turn below.
Figure 2: Primary Sections
Background, Intro. > Non-Results > Results > Discussion > Conclusion > Exec. Summary
Background and Introduction
When available, utilize any state-of-the-art analysis of the product/therapy from the protocol for your CSR introduction. If not available, you can briefly summarize the study design, objectives, and population and then you’ll need to craft a novel but brief state-of-the-art analysis based on literature review.
Be sure to align with the key messaging of your study and the indications of your study drug, device, or other type of therapeutic product or method. Utilize good literature review practices, such as choosing peer-reviewed publications, editorials from key opinion leaders in the therapeutic area, and studies with large or randomized cohorts, for support. This section will likely be no longer than one page.
Non-Results Section
Whether to cut and paste the procedures and assessments, primary and secondary endpoints, parameters or hypotheses, planned statistical analyses, monitoring plans, adverse event definitions, and assessment rules directly from the protocol or to simply refer to the protocol and the other study documents in an appendix is a topic of debate amongst medical writers of CSRs. Keep in mind that the CSR should be able to stand alone as a document, and thus while it is important to keep the document concise, it must be comprehensive enough for the reader to understand the study design, objectives, endpoints, processes, and intended analyses without having to refer constantly to the protocol. Regardless, in any summary of the study design, processes, and endpoints, be sure to align with any previously utilized language for consistency across study documents.
Results Section
Using the template and your tables as your structure, summarize the data and pull out any signals and trends, aligning with key messaging where possible. Start with patient disposition and demographics as per the template. Note any protocol deviations that may or may not have impacted patient safety or the evaluation of the outcomes.
Assess and evaluate the study outcome results against primary endpoints and secondary endpoints before discussing any additional secondary outcomes. You should not simply restate the data in the tables; however, refer to specifics in the tables when summarizing.
If you find that you cannot make a statement or conclusion given the TLFs you have, or you are consistently having to perform your own math to support your statements, consider asking your biostatistician to create the tables that will represent the data in a way that will better support your statement. For instance, it is acceptable to state that “most” of the patients responded to the study drug if more than 50% did so; however, if you are having to consistently add up percentages in a table to be able to state, for example, that 77% of the patients responded in a certain way and 33% responded in another, then you should have the biostatistician reformat the data output so it represents the percentages you want to report.
Patient narratives are an important source of context for the reader of the CSR. Depending on your study, you may need to collaborate with either your teammates responsible for assessment of adverse events or the study database administrator to help generate patient and/or event narratives for the CSR. If tasked with compiling or editing patient narratives yourself, the ICH E3 guideline prescribes the necessary components of a comprehensive patient safety narrative (Section 12).{2}
Narrative writing advice has also been previously published and would be a helpful source of direction for the novice narrative writer.{9,10} Narratives are suggested for every patient who experienced a safety endpoint event or death during the course of the study. Tie in patient narratives where appropriate when discussing safety events or refer to the patient narrative section when highlighting a particular patient’s data.
Discussions and Conclusions
Discussion and conclusion sections can either be placed after each section or placed at the end of the document. They should not simply restate the previous table summaries, but provide context and align the results with key messaging. Use an evidence-based approach, including literature references to provide more context as to the nature of the study outcomes with respect to the state of the art for the product/therapy, outcomes from alternate approaches, or further justification of clinical benefit with regard to potential disease progression. The conclusion section at the end of the document is often in bulleted format—not only for ease of the reader, but also to clearly highlight the key messaging and important outcomes you wish to impart.
Executive Summary
The executive summary, while placed at the front of the document prior to the introduction, is often easiest to construct last, as an overall summary of the entire document. The key elements of this summary should briefly recap the study design and objectives. Most likely only the primary and secondary endpoints should be included, unless additional outcomes proved compelling and important within the course of the study. Refer to any important literature comparisons as they relate to any conclusions made about the success or outcomes of the trials. Conclude the executive summary in a similar fashion to the overall study conclusion.
Sidebar: Tips and Tricks for the CSR Writing Process
Review Process
The review process can either facilitate a better document or it can slow down the entire process. The purpose of a cross functional review of a CSR is to confirm accurate key study messaging and data; allow medical review of the patient narratives, outcomes, and conclusionary statements; review the logical flow of ideas; and ensure that the CSR language is consistent across any other study document (i.e., the protocol, statistical analysis plan, etc.).
Sidebar: Tips and Tricks for an Efficient Review Process
CSRs are required by regulatory authorities to report and summarize the outcomes of a clinical study. Pre-project stakeholder determination and timeline planning can help with project management. Templates contained with the ICH E3 guideline can help organize the project as well as help create and finalize a document that is fit for purpose and meets the content expectations of the regulatory reviewer.
- ICH Working Group. 2016. ICH HARMONISED GUIDELINE INTEGRATED ADDENDUM TO ICH E6(R1): GUIDELINE FOR GOOD CLINICAL PRACTICE E6(R2).
- ICH Working Group. 1995. ICH HARMONISED TRIPARTITE GUIDELINE: Structure and Content of Clinical Study Reports E3 .
- U.S. Department of Health and Human Services. 2016. Clinical Trials Registration and Results Information Submission, 42 CFR Part 11. https://www.federalregister.gov/documents/2016/09/21/2016-22129/clinical-trials-registration-and-results-information-submission
- European Commission. 2001. Letter to Stakeholders Regarding the Requirements to provide results for Authortied clinical trials in EUDRACT. In: Article 57(2) Regulation (EC) No 726/2004 and Article 41(2) of Regulation (EC) No 1901/2006. https://eudract.ema.europa.eu/
- U.S. Food and Drug Administration. 2018. ICH Guidance Documents . https://www.fda.gov/science-research/guidance-documents-including-information-sheets-and-notices/ich-guidance-documents
- Transcelerate Biopharma Inc. Clinical Template Suite (CTS), Template, Resources, and Use Guidance. https://transceleratebiopharmainc.com/assets/clinical-content-reuse-assets/
- Hamilton S, Bernstein AB, Blakey G, et al. 2016. Developing the Clarity and Openness in Reporting: E3-based (CORE) Reference user manual for creation of clinical study reports in the era of clinical trial transparency. Research integrity and peer review. 1:4.
- Hamilton S. 2014. Effective authoring of clinical study reports. Medical Writing 23(2).
- Nambiar I. 2018. Analysis of serious adverse event: Writing a narrative. Perspect Clin Res 9(2):103–6.
- Ledade SD, Jain SN, Darji AA, Gupta VH. 2017. Narrative writing: Effective ways and best practices. Perspect Clin Res 8(2):58–62.

Sheryl Stewart, MCR, CCRP, ( [email protected] ) is a Medical Writer working in the medical device industry in southern California.
Sorry, we couldn't find any jobs that match your criteria.

Barriers to Clinical Trial Enrollment: Focus on Underrepresented Populations

Using Simulation to Teach Research

An Approach to a Benefit-Risk Framework

Trip: Overview
- Get Individual Help
Trip Overview
Trip is a clinical search engine designed to allow users to quickly and easily find a variety of high-quality research evidence to support their practice and/or care. Results provided can be sorted by quality, relevance, date or popularity and organized by the type of evidence via a pyramid graphic under the title of each article.

The library provides the free version of Trip. We do not have an institutional subscription to this product. There is a pro version of Trip that can be purchased which allows more full text results and additional tools if you wish to subscribe on your own. The personal subscription has an annual cost of $55 + tax.
For a comparison of free versus paid features see: Trip Feature Comparison
Special Features

- Trip provides a graphic next to each result to inform you about level of evidence it qualifies as. The hierarchy is shown above.
- Filtering options are provided to focus on the kind of evidence (systematic reviews, primary research, regulatory guidance, etc.) desired.
- Search results can be ranked by quality, relevance, date or popularity.
- Patient/population, Intervention, Comparison and Outcomes ( PICO) searching is available allowing you to focus in on your research question.
- Trip provides a large collection of clinical guidelines and thousands of systematic reviews.
- The SmartSearch feature displays three related articles.
- If you sign up with your email to Trip, you can receive alerts about newly added sources regarding topics of interest to you at no charge.
Trip has a blog and the most recent posts are shown here. Click on the website link below to see the rest.
- Next: Tutorials >>
- Last Updated: Sep 8, 2023 8:01 AM
- URL: https://library.aah.org/guides/trip
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- Current issue
- BMJ Journals More You are viewing from: Google Indexer
You are here
- Volume 7, Issue 4
TRIP database
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- Victor M Montori , MD, MSc ,
- Jon O Ebbert , MD
- Mayo Clinic Rochester, Minnesota, USA
https://doi.org/10.1136/ebm.7.4.104
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
TRIP is easy to use, with a simple web page design that provides quick downloads even with slow internet connections. TRIP organises the links in its database into clinical areas (eg, cancer, cardiovascular, pregnancy and childbirth, and mental health) and features a self directed learning area that offers online continuing medical education based on systematic reviews. A user-friendly search engine is also available on the site. The user can enter a word or phrase (searched exactly as typed) or a simple Boolean expression into a query box. The search is limited to the title of the link or to the title and text that were captured in the database when the hit was first identified by TRIP (this text could include the executive summary of a guideline or the abstract of a manuscript). A helpful troubleshooting guide to improve searching is a click away. Users can store their search terms to be run periodically and can have the results emailed.
The search results are organised into colour coded categories. The categories include evidence-based resources (including links to ACP Journal Club and Evidence Based Medicine and links to databanks of critically appraised topics); query-answering services (mainly the Ask TRIP to Rapidly Alleviate Confused Thoughts [ATTRACT] service, www.attract.wales.nhs.uk ); peer reviewed journals (including the journals published by the BMJ Publishing Group, JAMA , Annals of Internal Medicine , and the New England Journal of Medicine ); guidelines; electronic textbooks (including the Merck Manual and emedicine.com); and links to the Clinical Queries feature of PubMed ( www.pubmed.gov .). Within each of these categories the links are listed by date of publication and include the name of the host website.
To evaluate TRIP, we completed a series of searches using questions that arose during our clinical practice. A search that used the simple Boolean expression “diabetes AND smoking” and that was restricted to “titles” identified 6 hits: 5 articles in peer reviewed journals and 1 guideline from the American Diabetes Association Practice Recommendations. When we completed the search using “titles and text,” it took longer, and most of the 387 resulting hits were not relevant.
A search for “vitamin E” in “titles” led to 19 hits. The results included critically appraised topics of several randomised trials of vitamin E and 3 Cochrane reviews. The hits also included ATTRACT’s answer to a question about the effectiveness of vitamin E in dementia. The peer reviewed journal hits included abstracts to 3 important clinical trials. The electronic textbook hits offered links to narrative reviews of vitamin E toxicity and deficiency.
Unfortunately, the search engine does not enhance the search terms entered by users who type in synonyms or medical subject headings. We found that searching by title and text yielded too many irrelevant hits, but this finding may represent our lack of experience with the search engine. Performance of quick searches on specific topics using TRIP can be hit or miss because success is dependent on the content of the database. We feel that the inclusion of textbooks delays the search and provides information of limited validity and clinical usefulness.
TRIP offers a friendly interface and quick access to the evidence (particularly if the search is limited to titles) with user friendly organisation of search results. We recommend this resource for those seeking pre-appraised evidence, reviews, and guidelines. Those seeking the original studies may be better off using the Clinical Queries and Related Articles functions in PubMed.
Methods/Quality:★☆☆☆☆
The website can be found at http://www.userguides.org
Read the full text or download the PDF:
Trip Database Blog
Liberating the literature, latest evidence from trip.
At Trip, we continuously update our database with thousands of new articles each month, prioritizing the inclusion of high-quality evidence such as guidelines and systematic reviews. Our ‘Latest’ feature is designed to present these recent additions in a user-centric manner, tailored according to individual profiles. This customization occurs in two primary ways:
- Clinical area: For users with specific clinical interests noted in their profiles, such as Gastroenterology, we curate and list the most recent, relevant evidence in that field. ( Click here to explore the latest articles in Gastroenterology).
- Personalised Topics: Users have the option to highlight particular topics of interest within their profiles. Based on these selections, we actively search for and recommend new articles that align with these specified interests, such as research on bipolar .
To ensure our users remain informed of the latest evidence, we distribute a monthly email containing a direct link to these updated resources. This link is accessible at any time, allowing users the flexibility to explore the latest findings at their convenience. While this feature is frequently utilized and appreciated by our community, we believe there are opportunities for enhancement to further enrich user engagement and satisfaction.

At the top is a list of 5 ‘key’ documents and below that a list of recorded search terms and/or clinical areas of interest. If you click on a link it takes you to a page of search results – the results being a search for the topic for content added that month:

Recognizing the current approach as ‘sub-optimal’—a diplomatic term for inadequate—we are in the process of re-evaluating this feature. It’s evident that users have a strong desire to access the most recent evidence related to their specific areas of interest, and we acknowledge the need for improvement in meeting this demand. We highly value your feedback and encourage you to share your insights and suggestions either through the comments section or by directly emailing me at [email protected] . This initiative is part of our ongoing commitment to enhance user experience and optimize our services for better search engine visibility and user satisfaction.
Share this:
- Uncategorized
Leave a comment Cancel reply
Recent posts.
- Relevancy – a big change
- We’ve moved
- Using document clustering to show evolution of a topic area
- Clustering search results using Carrot2
- Moving to the cloud
Recent Comments
- February 2024
- January 2024
- December 2023
- November 2023
- October 2023
- September 2023
- August 2023
- February 2023
- January 2023
- December 2022
- November 2022
- October 2022
- September 2022
- August 2022
- February 2022
- January 2022
- December 2021
- November 2021
- October 2021
- September 2021
- August 2021
- February 2021
- December 2020
- October 2020
- September 2020
- February 2020
- January 2020
- December 2019
- November 2019
- October 2019
- September 2019
- August 2019
- February 2019
- January 2019
- December 2018
- November 2018
- October 2018
- September 2018
- August 2018
- February 2018
- January 2018
- November 2017
- October 2017
- September 2017
- August 2017
- February 2017
- January 2017
- December 2016
- November 2016
- October 2016
- September 2016
- August 2016
- February 2016
- January 2016
- December 2015
- November 2015
- October 2015
- September 2015
- August 2015
- February 2015
- January 2015
- December 2014
- November 2014
- October 2014
- September 2014
- August 2014
- February 2014
- January 2014
- December 2013
- November 2013
- October 2013
- August 2013
- February 2013
- January 2013
- December 2012
- November 2012
- October 2012
- September 2012
- August 2012
- January 2012
- December 2011
- November 2011
- October 2011
- September 2011
- August 2011
- February 2011
- January 2011
- December 2010
- November 2010
- October 2010
- September 2010
- August 2010
- February 2010
- January 2010
- December 2009
- November 2009
- October 2009
- September 2009
- August 2009
- February 2009
- January 2009
- December 2008
- November 2008
- October 2008
- September 2008
- August 2008
- February 2008
- January 2008
- December 2007
- November 2007
- October 2007
- September 2007
- August 2007
- February 2007
- January 2007
- December 2006
- November 2006
- October 2006
- September 2006
- August 2006
- February 2006
- Advanced search
- answer engine
- binary clock
- broken links
- clickstream
- clinical areas
- clinical decisions
- clinical trials
- communication
- critical friend
- development
- evidence hierarchy
- evidence live
- evidence service
- excluding results
- filter bubbles
- flibanserin
- google maps
- half man half biscuit
- http://schemas.google.com/blogger/2008/kind#post
- Iain Chalmers
- improved search
- institutions
- machine learning
- medical images
- multi-lingual
- new features
- nhs choices
- open access
- open medicine
- osteoporosis
- peer-review
- personalised search
- professions
- publication
- publication bias
- question analysis
- rapid reviews
- registration
- search algorithm
- search refinement
- search safety net
- search terms
- semantic annotation
- sentiment analysis
- serendipity
- strange results
- systematic review methods
- systematic reviews
- top articles
- trial registries
- TRIP Evidence Reviews
- trip profile
- uncertainties
- user-added content
Blog at WordPress.com.
- Already have a WordPress.com account? Log in now.
- Subscribe Subscribed
- Copy shortlink
- Report this content
- View post in Reader
- Manage subscriptions
- Collapse this bar
Preparing Monitors for Tomorrow’s Clinical Trials
In the first blog in our series, we discussed how the global pandemic facilitated the need for the industry to change the way clinical trials are run. In this blog, we’ll explore the role of the CRA in a post-COVID era.
Monitoring Challenges
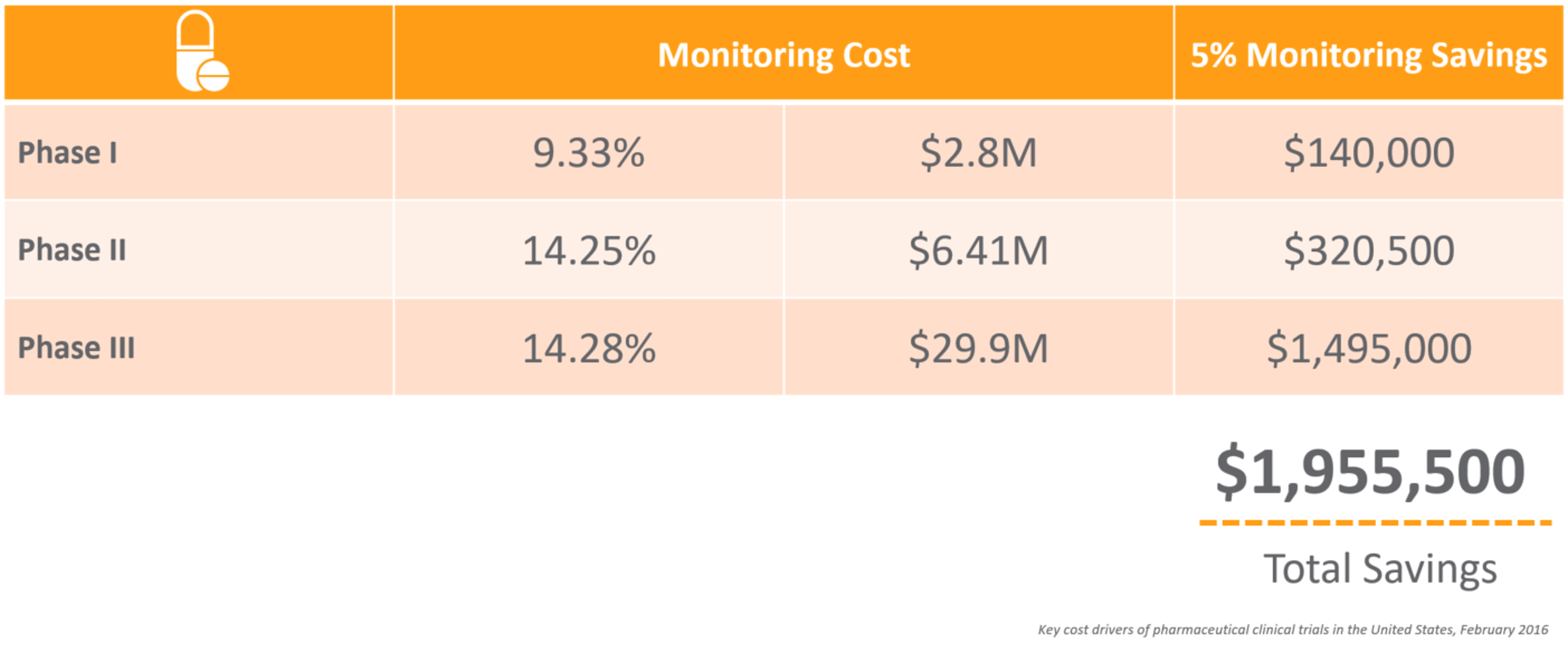
CRAs have a difficult job – from the extensive travel to the tedious time spent preparing for onsite visits and completing trip reports. For an average phase III trial for large pharma, monitors can spend up to 18 hours on just this task alone. With many top 20 pharma averaging over 40,000 monitoring trip reports per year, 1 it’s not surprising that 25%-30% of total clinical trial costs are attributed to site monitoring. 2
The time spent on monitoring visits is compounded by the reality that many CRAs work across 10+ systems, which are often siloed, adding effort and complexity to an already tough job.
Monitoring is an area ripe for optimization. Suppose you could reduce the time, effort, and cost of monitoring by 5%. What impact would that have on your clinical development costs and time to market?
Streamlining Clinical Trials: Adopting a Risk-Based Approach
One way to achieve significant time and cost savings, and increase study quality, is a sound RBM strategy that incorporates quality by design and centralized remote monitoring. Focusing on risks specific to a study’s critical data allows sponsors and CROs to be more productive and allocate resources more effectively. This risk-based approach also conforms with ICH GCP guidelines, enabling faster processes and workflows, and improving study quality.
By identifying critical data, performing risk assessments, and providing a more targeted SDV and remote monitoring strategy, clinical operations leaders can better mitigate risks and optimize trial outcomes.
The Shift in the Monitor’s Role
COVID has demonstrated just how much work CRAs can do offsite, such as reviewing regulatory documents and statistical reports. Armed with highly focused reports if they must go onsite, monitors can dedicate their time to value-add activities – ensuring drug storage procedures align with the protocol, supporting sites by creating an enrollment strategy, fixing known issues, and building stronger relationships with site personnel.
If there is a silver lining to this unprecedented global event, it’s that the industry has proven we can execute remote trials without 100% SDV, while upholding the same stringent efficacy, data quality, and data integrity standards. This approach has resulted in the first COVID vaccines being developed and delivered at record speeds. It will be interesting to see if the learnings we gained during this unprecedented time carry forward into normal monitoring processes post-COVID.
Check out the next blog in our series, where we dive into how to garner internal support for an RBM initiative and steps you can take to ensure a successful deployment and adoption.
1 De-identified average from Veeva customers. 2 Branch, E. (2016, April 30). Ways to Lower Costs of Clinical Trials and How CROs Help. Retrieved January 21, 2019, from https://www.americanpharmaceuticalreview.com/Featured-Articles/185929-Ways-to-Lower-Costs-of-Clinical-Trials-and-How-CROs-Help/
Interested in learning more about how Veeva can help?
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- v.15(2); 2023 Feb
- PMC10023071

Clinical Trials and Clinical Research: A Comprehensive Review
Venkataramana kandi.
1 Clinical Microbiology, Prathima Institute of Medical Sciences, Karimnagar, IND
Sabitha Vadakedath
2 Biochemistry, Prathima Institute of Medical Sciences, Karimnagar, IND
Clinical research is an alternative terminology used to describe medical research. Clinical research involves people, and it is generally carried out to evaluate the efficacy of a therapeutic drug, a medical/surgical procedure, or a device as a part of treatment and patient management. Moreover, any research that evaluates the aspects of a disease like the symptoms, risk factors, and pathophysiology, among others may be termed clinical research. However, clinical trials are those studies that assess the potential of a therapeutic drug/device in the management, control, and prevention of disease. In view of the increasing incidences of both communicable and non-communicable diseases, and especially after the effects that Coronavirus Disease-19 (COVID-19) had on public health worldwide, the emphasis on clinical research assumes extremely essential. The knowledge of clinical research will facilitate the discovery of drugs, devices, and vaccines, thereby improving preparedness during public health emergencies. Therefore, in this review, we comprehensively describe the critical elements of clinical research that include clinical trial phases, types, and designs of clinical trials, operations of trial, audit, and management, and ethical concerns.
Introduction and background
A clinical trial is a systematic process that is intended to find out the safety and efficacy of a drug/device in treating/preventing/diagnosing a disease or a medical condition [ 1 , 2 ]. Clinical trial includes various phases that include phase 0 (micro-dosing studies), phase 1, phase 2, phase 3, and phase 4 [ 3 ]. Phase 0 and phase 2 are called exploratory trial phases, phase 1 is termed the non-therapeutic phase, phase 3 is known as the therapeutic confirmatory phase, and phase 4 is called the post-approval or the post-marketing surveillance phase. Phase 0, also called the micro-dosing phase, was previously done in animals but now it is carried out in human volunteers to understand the dose tolerability (pharmacokinetics) before being administered as a part of the phase 1 trial among healthy individuals. The details of the clinical trial phases are shown in Table Table1 1 .
This table has been created by the authors.
MTD: maximum tolerated dose; SAD: single ascending dose; MAD: multiple ascending doses; NDA: new drug application; FDA: food and drug administration
Clinical research design has two major types that include non-interventional/observational and interventional/experimental studies. The non-interventional studies may have a comparator group (analytical studies like case-control and cohort studies), or without it (descriptive study). The experimental studies may be either randomized or non-randomized. Clinical trial designs are of several types that include parallel design, crossover design, factorial design, randomized withdrawal approach, adaptive design, superiority design, and non-inferiority design. The advantages and disadvantages of clinical trial designs are depicted in Table Table2 2 .
There are different types of clinical trials that include those which are conducted for treatment, prevention, early detection/screening, and diagnosis. These studies address the activities of an investigational drug on a disease and its outcomes [ 4 ]. They assess whether the drug is able to prevent the disease/condition, the ability of a device to detect/screen the disease, and the efficacy of a medical test to diagnose the disease/condition. The pictorial representation of a disease diagnosis, treatment, and prevention is depicted in Figure Figure1 1 .

This figure has been created by the authors.
The clinical trial designs could be improvised to make sure that the study's validity is maintained/retained. The adaptive designs facilitate researchers to improvise during the clinical trial without interfering with the integrity and validity of the results. Moreover, it allows flexibility during the conduction of trials and the collection of data. Despite these advantages, adaptive designs have not been universally accepted among clinical researchers. This could be attributed to the low familiarity of such designs in the research community. The adaptive designs have been applied during various phases of clinical trials and for different clinical conditions [ 5 , 6 ]. The adaptive designs applied during different phases are depicted in Figure Figure2 2 .

The Bayesian adaptive trial design has gained popularity, especially during the Coronavirus Disease-19 (COVID-19) pandemic. Such designs could operate under a single master protocol. It operates as a platform trial wherein multiple treatments can be tested on different patient groups suffering from disease [ 7 ].
In this review, we comprehensively discuss the essential elements of clinical research that include the principles of clinical research, planning clinical trials, practical aspects of clinical trial operations, essentials of clinical trial applications, monitoring, and audit, clinical trial data analysis, regulatory audits, and project management, clinical trial operations at the investigation site, the essentials of clinical trial experiments involving epidemiological, and genetic studies, and ethical considerations in clinical research/trials.
A clinical trial involves the study of the effect of an investigational drug/any other intervention in a defined population/participant. The clinical research includes a treatment group and a placebo wherein each group is evaluated for the efficacy of the intervention (improved/not improved) [ 8 ].
Clinical trials are broadly classified into controlled and uncontrolled trials. The uncontrolled trials are potentially biased, and the results of such research are not considered as equally as the controlled studies. Randomized controlled trials (RCTs) are considered the most effective clinical trials wherein the bias is minimized, and the results are considered reliable. There are different types of randomizations and each one has clearly defined functions as elaborated in Table Table3 3 .
Principles of clinical trial/research
Clinical trials or clinical research are conducted to improve the understanding of the unknown, test a hypothesis, and perform public health-related research [ 2 , 3 ]. This is majorly carried out by collecting the data and analyzing it to derive conclusions. There are various types of clinical trials that are majorly grouped as analytical, observational, and experimental research. Clinical research can also be classified into non-directed data capture, directed data capture, and drug trials. Clinical research could be prospective or retrospective. It may also be a case-control study or a cohort study. Clinical trials may be initiated to find treatment, prevent, observe, and diagnose a disease or a medical condition.
Among the various types of clinical research, observational research using a cross-sectional study design is the most frequently performed clinical research. This type of research is undertaken to analyze the presence or absence of a disease/condition, potential risk factors, and prevalence and incidence rates in a defined population. Clinical trials may be therapeutic or non-therapeutic type depending on the type of intervention. The therapeutic type of clinical trial uses a drug that may be beneficial to the patient. Whereas in a non-therapeutic clinical trial, the participant does not benefit from the drug. The non-therapeutic trials provide additional knowledge of the drug for future improvements. Different terminologies of clinical trials are delineated in Table Table4 4 .
In view of the increased cost of the drug discovery process, developing, and low-income countries depend on the production of generic drugs. The generic drugs are similar in composition to the patented/branded drug. Once the patent period is expired generic drugs can be manufactured which have a similar quality, strength, and safety as the patented drug [ 9 ]. The regulatory requirements and the drug production process are almost the same for the branded and the generic drug according to the Food and Drug Administration (FDA), United States of America (USA).
The bioequivalence (BE) studies review the absorption, distribution, metabolism, and excretion (ADME) of the generic drug. These studies compare the concentration of the drug at the desired location in the human body, called the peak concentration of the drug (Cmax). The extent of absorption of the drug is measured using the area under the receiver operating characteristic curve (AUC), wherein the generic drug is supposed to demonstrate similar ADME activities as the branded drug. The BE studies may be undertaken in vitro (fasting, non-fasting, sprinkled fasting) or in vivo studies (clinical, bioanalytical, and statistical) [ 9 ].
Planning clinical trial/research
The clinical trial process involves protocol development, designing a case record/report form (CRF), and functioning of institutional review boards (IRBs). It also includes data management and the monitoring of clinical trial site activities. The CRF is the most significant document in a clinical study. It contains the information collected by the investigator about each subject participating in a clinical study/trial. According to the International Council for Harmonisation (ICH), the CRF can be printed, optical, or an electronic document that is used to record the safety and efficacy of the pharmaceutical drug/product in the test subjects. This information is intended for the sponsor who initiates the clinical study [ 10 ].
The CRF is designed as per the protocol and later it is thoroughly reviewed for its correctness (appropriate and structured questions) and finalized. The CRF then proceeds toward the print taking the language of the participating subjects into consideration. Once the CRF is printed, it is distributed to the investigation sites where it is filled with the details of the participating subjects by the investigator/nurse/subject/guardian of the subject/technician/consultant/monitors/pharmacist/pharmacokinetics/contract house staff. The filled CRFs are checked for their completeness and transported to the sponsor [ 11 ].
Effective planning and implementation of a clinical study/trial will influence its success. The clinical study majorly includes the collection and distribution of the trial data, which is done by the clinical data management section. The project manager is crucial to effectively plan, organize, and use the best processes to control and monitor the clinical study [ 10 , 11 ].
The clinical study is conducted by a sponsor or a clinical research organization (CRO). A perfect protocol, time limits, and regulatory requirements assume significance while planning a clinical trial. What, when, how, and who are clearly planned before the initiation of a study trial. Regular review of the project using the bar and Gantt charts, and maintaining the timelines assume increased significance for success with the product (study report, statistical report, database) [ 10 , 11 ].
The steps critical to planning a clinical trial include the idea, review of the available literature, identifying a problem, formulating the hypothesis, writing a synopsis, identifying the investigators, writing a protocol, finding a source of funding, designing a patient consent form, forming ethics boards, identifying an organization, preparing manuals for procedures, quality assurance, investigator training and initiation of the trial by recruiting the participants [ 10 ].
The two most important points to consider before the initiation of the clinical trial include whether there is a need for a clinical trial, if there is a need, then one must make sure that the study design and methodology are strong for the results to be reliable to the people [ 11 ].
For clinical research to envisage high-quality results, the study design, implementation of the study, quality assurance in data collection, and alleviation of bias and confounding factors must be robust [ 12 ]. Another important aspect of conducting a clinical trial is improved management of various elements of clinical research that include human and financial resources. The role of a trial manager to make a successful clinical trial was previously reported. The trial manager could play a key role in planning, coordinating, and successfully executing the trial. Some qualities of a trial manager include better communication and motivation, leadership, and strategic, tactical, and operational skills [ 13 ].
Practical aspects of a clinical trial operations
There are different types of clinical research. Research in the development of a novel drug could be initiated by nationally funded research, industry-sponsored research, and clinical research initiated by individuals/investigators. According to the documents 21 code of federal regulations (CFR) 312.3 and ICH E-6 Good Clinical Practice (GCP) 1.54, an investigator is an individual who initiates and conducts clinical research [ 14 ]. The investigator plan, design, conduct, monitor, manage data, compile reports, and supervise research-related regulatory and ethical issues. To manage a successful clinical trial project, it is essential for an investigator to give the letter of intent, write a proposal, set a timeline, develop a protocol and related documents like the case record forms, define the budget, and identify the funding sources.
Other major steps of clinical research include the approval of IRBs, conduction and supervision of the research, data review, and analysis. Successful clinical research includes various essential elements like a letter of intent which is the evidence that supports the interest of the researcher to conduct drug research, timeline, funding source, supplier, and participant characters.
Quality assurance, according to the ICH and GCP guidelines, is necessary to be implemented during clinical research to generate quality and accurate data. Each element of the clinical research must have been carried out according to the standard operating procedure (SOP), which is written/determined before the initiation of the study and during the preparation of the protocol [ 15 ].
The audit team (quality assurance group) is instrumental in determining the authenticity of the clinical research. The audit, according to the ICH and GCP, is an independent and external team that examines the process (recording the CRF, analysis of data, and interpretation of data) of clinical research. The quality assurance personnel are adequately trained, become trainers if needed, should be good communicators, and must handle any kind of situation. The audits can be at the investigator sites evaluating the CRF data, the protocol, and the personnel involved in clinical research (source data verification, monitors) [ 16 ].
Clinical trial operations are governed by legal and regulatory requirements, based on GCPs, and the application of science, technology, and interpersonal skills [ 17 ]. Clinical trial operations are complex, time and resource-specific that requires extensive planning and coordination, especially for the research which is conducted at multiple trial centers [ 18 ].
Recruiting the clinical trial participants/subjects is the most significant aspect of clinical trial operations. Previous research had noted that most clinical trials do not meet the participant numbers as decided in the protocol. Therefore, it is important to identify the potential barriers to patient recruitment [ 19 ].
Most clinical trials demand huge costs, increased timelines, and resources. Randomized clinical trial studies from Switzerland were analyzed for their costs which revealed approximately 72000 USD for a clinical trial to be completed. This study emphasized the need for increased transparency with respect to the costs associated with the clinical trial and improved collaboration between collaborators and stakeholders [ 20 ].
Clinical trial applications, monitoring, and audit
Among the most significant aspects of a clinical trial is the audit. An audit is a systematic process of evaluating the clinical trial operations at the site. The audit ensures that the clinical trial process is conducted according to the protocol, and predefined quality system procedures, following GCP guidelines, and according to the requirements of regulatory authorities [ 21 ].
The auditors are supposed to be independent and work without the involvement of the sponsors, CROs, or personnel at the trial site. The auditors ensure that the trial is conducted by designated professionally qualified, adequately trained personnel, with predefined responsibilities. The auditors also ensure the validity of the investigational drug, and the composition, and functioning of institutional review/ethics committees. The availability and correctness of the documents like the investigational broacher, informed consent forms, CRFs, approval letters of the regulatory authorities, and accreditation of the trial labs/sites [ 21 ].
The data management systems, the data collection software, data backup, recovery, and contingency plans, alternative data recording methods, security of the data, personnel training in data entry, and the statistical methods used to analyze the results of the trial are other important responsibilities of the auditor [ 21 , 22 ].
According to the ICH-GCP Sec 1.29 guidelines the inspection may be described as an act by the regulatory authorities to conduct an official review of the clinical trial-related documents, personnel (sponsor, investigator), and the trial site [ 21 , 22 ]. The summary report of the observations of the inspectors is performed using various forms as listed in Table Table5 5 .
FDA: Food and Drug Administration; IND: investigational new drug; NDA: new drug application; IRB: institutional review board; CFR: code of federal regulations
Because protecting data integrity, the rights, safety, and well-being of the study participants are more significant while conducting a clinical trial, regular monitoring and audit of the process appear crucial. Also, the quality of the clinical trial greatly depends on the approach of the trial personnel which includes the sponsors and investigators [ 21 ].
The responsibility of monitoring lies in different hands, and it depends on the clinical trial site. When the trial is initiated by a pharmaceutical industry, the responsibility of trial monitoring depends on the company or the sponsor, and when the trial is conducted by an academic organization, the responsibility lies with the principal investigator [ 21 ].
An audit is a process conducted by an independent body to ensure the quality of the study. Basically, an audit is a quality assurance process that determines if a study is carried out by following the SPOs, in compliance with the GCPs recommended by regulatory bodies like the ICH, FDA, and other local bodies [ 21 ].
An audit is performed to review all the available documents related to the IRB approval, investigational drug, and the documents related to the patient care/case record forms. Other documents that are audited include the protocol (date, sign, treatment, compliance), informed consent form, treatment response/outcome, toxic response/adverse event recording, and the accuracy of data entry [ 22 ].
Clinical trial data analysis, regulatory audits, and project management
The essential elements of clinical trial management systems (CDMS) include the management of the study, the site, staff, subject, contracts, data, and document management, patient diary integration, medical coding, monitoring, adverse event reporting, supplier management, lab data, external interfaces, and randomization. The CDMS involves setting a defined start and finishing time, defining study objectives, setting enrolment and termination criteria, commenting, and managing the study design [ 23 ].
Among the various key application areas of clinical trial systems, the data analysis assumes increased significance. The clinical trial data collected at the site in the form of case record form is stored in the CDMS ensuring the errors with respect to the double data entry are minimized.
Clinical trial data management uses medical coding, which uses terminologies with respect to the medications and adverse events/serious adverse events that need to be entered into the CDMS. The project undertaken to conduct the clinical trial must be predetermined with timelines and milestones. Timelines are usually set for the preparation of protocol, designing the CRF, planning the project, identifying the first subject, and timelines for recording the patient’s data for the first visit.
The timelines also are set for the last subject to be recruited in the study, the CRF of the last subject, and the locked period after the last subject entry. The planning of the project also includes the modes of collection of the data, the methods of the transport of the CRFs, patient diaries, and records of severe adverse events, to the central data management sites (fax, scan, courier, etc.) [ 24 ].
The preparation of SOPs and the type and timing of the quality control (QC) procedures are also included in the project planning before the start of a clinical study. Review (budget, resources, quality of process, assessment), measure (turnaround times, training issues), and control (CRF collection and delivery, incentives, revising the process) are the three important aspects of the implementation of a clinical research project.
In view of the increasing complexity related to the conduct of clinical trials, it is important to perform a clinical quality assurance (CQA) audit. The CQA audit process consists of a detailed plan for conducting audits, points of improvement, generating meaningful audit results, verifying SOP, and regulatory compliance, and promoting improvement in clinical trial research [ 25 ]. All the components of a CQA audit are delineated in Table Table6 6 .
CRF: case report form; CSR: clinical study report; IC: informed consent; PV: pharmacovigilance; SAE: serious adverse event
Clinical trial operations at the investigator's site
The selection of an investigation site is important before starting a clinical trial. It is essential that the individuals recruited for the study meet the inclusion criteria of the trial, and the investigator's and patient's willingness to accept the protocol design and the timelines set by the regulatory authorities including the IRBs.
Before conducting clinical research, it is important for an investigator to agree to the terms and conditions of the agreement and maintain the confidentiality of the protocol. Evaluation of the protocol for the feasibility of its practices with respect to the resources, infrastructure, qualified and trained personnel available, availability of the study subjects, and benefit to the institution and the investigator is done by the sponsor during the site selection visit.
The standards of a clinical research trial are ensured by the Council for International Organizations of Medical Sciences (CIOMS), National Bioethics Advisory Commission (NBAC), United Nations Programme on Human Immunodeficiency Virus/Acquired Immunodeficiency Syndrome (HIV/AIDS) (UNAIDS), and World Medical Association (WMA) [ 26 ].
Recommendations for conducting clinical research based on the WMA support the slogan that says, “The health of my patient will be my first consideration.” According to the International Code of Medical Ethics (ICME), no human should be physically or mentally harmed during the clinical trial, and the study should be conducted in the best interest of the person [ 26 ].
Basic principles recommended by the Helsinki declaration include the conduction of clinical research only after the prior proof of the safety of the drug in animal and lab experiments. The clinical trials must be performed by scientifically, and medically qualified and well-trained personnel. Also, it is important to analyze the benefit of research over harm to the participants before initiating the drug trials.
The doctors may prescribe a drug to alleviate the suffering of the patient, save the patient from death, and gain additional knowledge of the drug only after obtaining informed consent. Under the equipoise principle, the investigators must be able to justify the treatment provided as a part of the clinical trial, wherein the patient in the placebo arm may be harmed due to the unavailability of the therapeutic/trial drug.
Clinical trial operations greatly depend on the environmental conditions and geographical attributes of the trial site. It may influence the costs and targets defined by the project before the initiation. It was noted that one-fourth of the clinical trial project proposals/applications submit critical data on the investigational drug from outside the country. Also, it was noted that almost 35% of delays in clinical trials owing to patient recruitment with one-third of studies enrolling only 5% of the participants [ 27 ].
It was suggested that clinical trial feasibility assessment in a defined geographical region may be undertaken for improved chances of success. Points to be considered under the feasibility assessment program include if the disease under the study is related to the population of the geographical region, appropriateness of the study design, patient, and comparator group, visit intervals, potential regulatory and ethical challenges, and commitments of the study partners, CROs in respective countries (multi-centric studies) [ 27 ].
Feasibility assessments may be undertaken at the program level (ethics, regulatory, and medical preparedness), study level (clinical, regulatory, technical, and operational aspects), and at the investigation site (investigational drug, competency of personnel, participant recruitment, and retention, quality systems, and infrastructural aspects) [ 27 ].
Clinical trials: true experiments
In accordance with the revised schedule "Y" of the Drugs and Cosmetics Act (DCA) (2005), a drug trial may be defined as a systematic study of a novel drug component. The clinical trials aim to evaluate the pharmacodynamic, and pharmacokinetic properties including ADME, efficacy, and safety of new drugs.
According to the drug and cosmetic rules (DCR), 1945, a new chemical entity (NCE) may be defined as a novel drug approved for a disease/condition, in a specified route, and at a particular dosage. It also may be a new drug combination, of previously approved drugs.
A clinical trial may be performed in three types; one that is done to find the efficacy of an NCE, a comparison study of two drugs against a medical condition, and the clinical research of approved drugs on a disease/condition. Also, studies of the bioavailability and BE studies of the generic drugs, and the drugs already approved in other countries are done to establish the efficacy of new drugs [ 28 ].
Apart from the discovery of a novel drug, clinical trials are also conducted to approve novel medical devices for public use. A medical device is defined as any instrument, apparatus, appliance, software, and any other material used for diagnostic/therapeutic purposes. The medical devices may be divided into three classes wherein class I uses general controls; class II uses general and special controls, and class III uses general, special controls, and premarket approvals [ 28 ].
The premarket approval applications ensure the safety and effectiveness, and confirmation of the activities from bench to animal to human clinical studies. The FDA approval for investigational device exemption (IDE) for a device not approved for a new indication/disease/condition. There are two types of IDE studies that include the feasibility study (basic safety and potential effectiveness) and the pivotal study (trial endpoints, randomization, monitoring, and statistical analysis plan) [ 28 ].
As evidenced by the available literature, there are two types of research that include observational and experimental research. Experimental research is alternatively known as the true type of research wherein the research is conducted by the intervention of a new drug/device/method (educational research). Most true experiments use randomized control trials that remove bias and neutralize the confounding variables that may interfere with the results of research [ 28 ].
The variables that may interfere with the study results are independent variables also called prediction variables (the intervention), dependent variables (the outcome), and extraneous variables (other confounding factors that could influence the outside). True experiments have three basic elements that include manipulation (that influence independent variables), control (over extraneous influencers), and randomization (unbiased grouping) [ 29 ].
Experiments can also be grouped as true, quasi-experimental, and non-experimental studies depending on the presence of specific characteristic features. True experiments have all three elements of study design (manipulation, control, randomization), and prospective, and have great scientific validity. Quasi-experiments generally have two elements of design (manipulation and control), are prospective, and have moderate scientific validity. The non-experimental studies lack manipulation, control, and randomization, are generally retrospective, and have low scientific validity [ 29 ].
Clinical trials: epidemiological and human genetics study
Epidemiological studies are intended to control health issues by understanding the distribution, determinants, incidence, prevalence, and impact on health among a defined population. Such studies are attempted to perceive the status of infectious diseases as well as non-communicable diseases [ 30 ].
Experimental studies are of two types that include observational (cross-sectional studies (surveys), case-control studies, and cohort studies) and experimental studies (randomized control studies) [ 3 , 31 ]. Such research may pose challenges related to ethics in relation to the social and cultural milieu.
Biomedical research related to human genetics and transplantation research poses an increased threat to ethical concerns, especially after the success of the human genome project (HGP) in the year 2000. The benefits of human genetic studies are innumerable that include the identification of genetic diseases, in vitro fertilization, and regeneration therapy. Research related to human genetics poses ethical, legal, and social issues (ELSI) that need to be appropriately addressed. Most importantly, these genetic research studies use advanced technologies which should be equally available to both economically well-placed and financially deprived people [ 32 ].
Gene therapy and genetic manipulations may potentially precipitate conflict of interest among the family members. The research on genetics may be of various types that include pedigree studies (identifying abnormal gene carriers), genetic screening (for diseases that may be heritable by the children), gene therapeutics (gene replacement therapy, gene construct administration), HGP (sequencing the whole human genome/deoxyribonucleic acid (DNA) fingerprinting), and DNA, cell-line banking/repository [ 33 ]. The biobanks are established to collect and store human tissue samples like umbilical tissue, cord blood, and others [ 34 ].
Epidemiological studies on genetics are attempts to understand the prevalence of diseases that may be transmitted among families. The classical epidemiological studies may include single case observations (one individual), case series (< 10 individuals), ecological studies (population/large group of people), cross-sectional studies (defined number of individuals), case-control studies (defined number of individuals), cohort (defined number of individuals), and interventional studies (defined number of individuals) [ 35 ].
Genetic studies are of different types that include familial aggregation (case-parent, case-parent-grandparent), heritability (study of twins), segregation (pedigree study), linkage study (case-control), association, linkage, disequilibrium, cohort case-only studies (related case-control, unrelated case-control, exposure, non-exposure group, case group), cross-sectional studies, association cohort (related case-control, familial cohort), and experimental retrospective cohort (clinical trial, exposure, and non-exposure group) [ 35 ].
Ethics and concerns in clinical trial/research
Because clinical research involves animals and human participants, adhering to ethics and ethical practices assumes increased significance [ 36 ]. In view of the unethical research conducted on war soldiers after the Second World War, the Nuremberg code was introduced in 1947, which promulgated rules for permissible medical experiments on humans. The Nuremberg code suggests that informed consent is mandatory for all the participants in a clinical trial, and the study subjects must be made aware of the nature, duration, and purpose of the study, and potential health hazards (foreseen and unforeseen). The study subjects should have the liberty to withdraw at any time during the trial and to choose a physician upon medical emergency. The other essential principles of clinical research involving human subjects as suggested by the Nuremberg code included benefit to the society, justification of study as noted by the results of the drug experiments on animals, avoiding even minimal suffering to the study participants, and making sure that the participants don’t have life risk, humanity first, improved medical facilities for participants, and suitably qualified investigators [ 37 ].
During the 18th world medical assembly meeting in the year 1964, in Helsinki, Finland, ethical principles for doctors practicing research were proposed. Declaration of Helsinki, as it is known made sure that the interests and concerns of the human participants will always prevail over the interests of the society. Later in 1974, the National Research Act was proposed which made sure that the research proposals are thoroughly screened by the Institutional ethics/Review Board. In 1979, the April 18th Belmont report was proposed by the national commission for the protection of human rights during biomedical and behavioral research. The Belmont report proposed three core principles during research involving human participants that include respect for persons, beneficence, and justice. The ICH laid down GCP guidelines [ 38 ]. These guidelines are universally followed throughout the world during the conduction of clinical research involving human participants.
ICH was first founded in 1991, in Brussels, under the umbrella of the USA, Japan, and European countries. The ICH conference is conducted once every two years with the participation from the member countries, observers from the regulatory agencies, like the World Health Organization (WHO), European Free Trade Association (EFTA), and the Canadian Health Protection Branch, and other interested stakeholders from the academia and the industry. The expert working groups of the ICH ensure the quality, efficacy, and safety of the medicinal product (drug/device). Despite the availability of the Nuremberg code, the Belmont Report, and the ICH-GCP guidelines, in the year 1982, International Ethical Guidelines for Biomedical Research Involving Human Subjects was proposed by the CIOMS in association with WHO [ 39 ]. The CIOMS protects the rights of the vulnerable population, and ensures ethical practices during clinical research, especially in underdeveloped countries [ 40 ]. In India, the ethical principles for biomedical research involving human subjects were introduced by the Indian Council of Medical Research (ICMR) in the year 2000 and were later amended in the year 2006 [ 41 ]. Clinical trial approvals can only be done by the IRB approved by the Drug Controller General of India (DGCI) as proposed in the year 2013 [ 42 ].
Current perspectives and future implications
A recent study attempted to evaluate the efficacy of adaptive clinical trials in predicting the success of a clinical trial drug that entered phase 3 and minimizing the time and cost of drug development. This study highlighted the drawbacks of such clinical trial designs that include the possibility of type 1 (false positive) and type 2 (false negative) errors [ 43 ].
The usefulness of animal studies during the preclinical phases of a clinical trial was evaluated in a previous study which concluded that animal studies may not completely guarantee the safety of the investigational drug. This is noted by the fact that many drugs which passed toxicity tests in animals produced adverse reactions in humans [ 44 ].
The significance of BE studies to compare branded and generic drugs was reported previously. The pharmacokinetic BE studies of Amoxycillin comparing branded and generic drugs were carried out among a group of healthy participants. The study results have demonstrated that the generic drug had lower Cmax as compared to the branded drug [ 45 ].
To establish the BE of the generic drugs, randomized crossover trials are carried out to assess the Cmax and the AUC. The ratio of each pharmacokinetic characteristic must match the ratio of AUC and/or Cmax, 1:1=1 for a generic drug to be considered as a bioequivalent to a branded drug [ 46 ].
Although the generic drug development is comparatively more beneficial than the branded drugs, synthesis of extended-release formulations of the generic drug appears to be complex. Since the extended-release formulations remain for longer periods in the stomach, they may be influenced by gastric acidity and interact with the food. A recent study suggested the use of bio-relevant dissolution tests to increase the successful production of generic extended-release drug formulations [ 47 ].
Although RCTs are considered the best designs, which rule out bias and the data/results obtained from such clinical research are the most reliable, RCTs may be plagued by miscalculation of the treatment outcomes/bias, problems of cointerventions, and contaminations [ 48 ].
The perception of healthcare providers regarding branded drugs and their view about the generic equivalents was recently analyzed and reported. It was noted that such a perception may be attributed to the flexible regulatory requirements for the approval of a generic drug as compared to a branded drug. Also, could be because a switch from a branded drug to a generic drug in patients may precipitate adverse events as evidenced by previous reports [ 49 ].
Because the vulnerable population like drug/alcohol addicts, mentally challenged people, children, geriatric age people, military persons, ethnic minorities, people suffering from incurable diseases, students, employees, and pregnant women cannot make decisions with respect to participating in a clinical trial, ethical concerns, and legal issues may prop up, that may be appropriately addressed before drug trials which include such groups [ 50 ].
Conclusions
Clinical research and clinical trials are important from the public health perspective. Clinical research facilitates scientists, public health administrations, and people to increase their understanding and improve preparedness with reference to the diseases prevalent in different geographical regions of the world. Moreover, clinical research helps in mitigating health-related problems as evidenced by the current Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) pandemic and other emerging and re-emerging microbial infections. Clinical trials are crucial to the development of drugs, devices, and vaccines. Therefore, scientists are required to be up to date with the process and procedures of clinical research and trials as discussed comprehensively in this review.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.

Clinical Trial Monitoring Reports and How to Write Them
Among the most important aspects of study is observation. Overseeing the advancement of any stage, measure, process, and procedure in real time is essential to the accurate conclusion of any clinical trial undertaking. Normal monitoring actions are needed to guarantee caliber, efficiency, compliance within predefined and regulations fundamentals. In addition, it guarantees comprehensiveness, and precision within clinical investigation. Such actions also ensure that the trial isn't just conducted in compliance with Standard Operating Procedures (SOPs). They must also function to validate it is correctly reported and recorded. Consider enrolling in the Clinical Research Coordinator course or the CRA training provided by CCRPS.
There demands something vital as a part in the execution of trials. And this thing is known as trial development reports.
Such a report ought to be carefully prepared and it must summarize the means that a study is done. It also ought to point out recruiting progress and procedures; should emphasize and clarify adjustments to the analysis, and ought to point security issues. If you're involved in such reporting or need an in-depth understanding of the procedures, the Advanced Clinical Research Project Manager Certification might be of interest.
Aside from the ethics committee, researchers could also need to present yearly improvement reports of an investigation (such as any applicable alterations or dangers) to spouses, encouraging associations, and/or organizations, along with other interested parties if needed. To understand more about these requirements and get certified, the ICH-GCP course is an excellent resource.
If you're thinking about getting important skills on GCP or you also would like to upgrade your own know-how, subscribe to our comprehensive Good Clinical Practice class here .
In summary, tracking and reporting processes is an incredibly significant function in clinical trials. The right conduct of these procedures ensure compliance with legislation, regulations, and predetermined conditions. In addition, they make certain that the study doesn't pose any dangers to wellbeing. Progress reports, subsequently, empower practitioners, specialists, researchers, ethics committees, as well as others involved to keep a tab on the trial and its own advancement. Clinical professionals need to signal any alterations or risks to react to them timely, correctly, and efficiently. For further training, consider the Pharmacovigilance Certification to deepen your knowledge in monitoring drug safety.
The objective of progress reports would be to accumulate and outline upgrades, key facets, along with summaries of a continuing trial. It's very crucial to be aware that progress reports must be filed to institutional evaluation board/independent integrity questionnaire (IRB/IEC), following a trial that has obtained positive opinion.
One other important issue to mention is there are many different forms and when it comes to submitting progress reports that researchers must take into consideration before proceeding.
Precisely, these kinds are:
Printing name and date of entry ought to be composed also. A digital copy is also needed to be delivered to the interested websites and committees inside a 30-day interval following the reporting procedure was completed.
The period of time whereby an advance report ought to be filed is at least one time in a year. Nevertheless, based upon the situation as well as the RECs' needs, these reports might be passed in more often, although the analysis is still going and till its official conclusion date.
Take courses from CCRPS and learn more on how to become a clinical research professional. For detailed guidance on submission requirements and processes, the Medical Monitor Certification and Advanced Principal Investigator Physician Certification can provide extensive knowledge.
Discover more from Clinical Trials Assistant Training | Clinical Research Training | Certified Clinical Research Professionals Course to further your career in clinical research management.

CRA Exam Questions
2018 clinical research associate (cra) salaries estimated from 1,850 american employees.
- Clinical Trial Management System Guide
- What’s New in This Release
9 Administering and Using Clinical Trip Reports
Administering and using clinical trip reports.
This chapter describes how to set up and use clinical trip reports in Siebel Clinical. It includes the following topics:
About Administering and Using Clinical Trip Reports
Scenario for managing clinical trip reports, creating questions for clinical trip reports using siebel smartscript, creating clinical trip report templates, applying clinical trip report templates, completing clinical trip reports, completing questionnaires for clinical trip reports, deleting unanswered questions from questionnaires of clinical trip reports, tracking case report forms, automated validation and notification messages for clinical trip reports, tracking completion status for clinical trip reports, tracking status accruals for clinical subjects of sites, viewing universal inbox notifications for action items of clinical trip reports, reviewing clinical trip reports, approving clinical trip reports, making clinical trip reports obsolete, creating new versions of clinical trip reports, viewing version information for clinical trip reports, viewing geographical location details for clinical trip reports, using audit trail for reviews and approvals of clinical trip reports, using audit trail for changes to clinical trip reports, generating oracle bi publisher reports for site visits.
The study managers or clinical administrators can set up templates for trip reports. The CRAs (clinical research associates) then use these templates when they create trip reports to record their visits to sites.
Advantages of using the trip reports in Siebel Clinical are:
Trip reports are consistent across the organization and are based on GCP (good clinical practice) and SOPs (standard operating procedures).
CRAs save time planning trips and writing trip reports.
Managers save time reviewing trip reports.
End users can generate a formatted, tamper-proof report for print or PDF from the trip report record.
A central repository exists for all trip reports.
This topic gives one example of how to manage clinical trip reports. You might manage clinical trip reports differently, depending on your business model.
This topic includes the following information:
Preparing Trip Report Templates
Preparing trip reports.
A clinical administrator prepares a set of trip report templates for the CRAs (clinical research associates) to use when preparing for and writing up their visits to clinical sites. For more information about site visits, see Creating and Managing Site Visits .
The clinical administrator prepares the following templates for each type of site visit the CRAs typically must perform:
Site evaluation
Site initiation
Site monitoring
Site close-out
The CRA (clinical research associate) is the end user of the Siebel Clinical product. Before visiting a site, the CRA uses the trip report to prepare for the visit. The follow-up items list reminds the CRA of the open activities from previous visits that the CRA can close.
After preparing a draft trip report, the CRA makes a hard copy of the report and takes this copy on the site visit. He can use the report as a reference to help keep track of the activities he completes while at the site.
After returning from a site visit, the CRA completes the trip report and generates a final report. He then submits this report to the study manager for approval. The manager reviews the report and approves it if it is satisfactory. If the manager approves the trip report, then it is then locked to prevent the CRA from making any further changes. If the trip report is not satisfactory, then the manager can reject the report and return it to the CRA for further attention.
You can use Siebel SmartScript to create questionnaires for clinical trip reports. For information about how to create questions using SmartScript, see Siebel SmartScript Administration Guide .
To add comments against questions in SmartScript, the following additional configuration is required:
You must create each comment as an individual question. Comments are always immediately followed by the corresponding question.
For each question that is intended to be a comment, enter the value ForceEnableQuestion.CommentForPrevQuestion in the Save User Parameters field when creating the question.
Some SmartScript question settings listed in Siebel SmartScript Administration Guide are not applicable for clinical trip reports. The following information lists the question settings that are not applicable to clinical trip reports.
You can assign a SmartScript questionnaire to a clinical trip report template after it is released using Siebel SmartScript. For information about how to release a script using Siebel SmartScript, see Siebel SmartScript Administration Guide .
Typically, the clinical administrator prepares a number of generic trip report templates, perhaps one designed for each of the different stages in the study. This topic describes how to create a clinical trip report. You can define additional activities, such as follow-up tasks to complete after the visit to the clinical site, in the Trip Report Template Details view.
To create a clinical trip report template
Navigate to the Administration - Clinical screen, then the Trip Report Templates view.
Create a new record and complete the necessary fields as shown in the following table.
Drill down on the Name field of the trip report template to display the Trip Report Template Details view.
Create new records to define each trip report activity.
The Template field of the Site Visits view displays all of the trip report templates that are applicable to protocol, region, and visit type details for that site visit. When you apply a trip report template to a trip report, all of the details in the template are copied to the trip report.
Checklist and follow-up activities in the Trip Report Template Details view of the trip report template are copied to the trip report, and overwrite any existing checklist and follow-up activities in the trip report.
SmartScript questionnaires in the SmartScript field of the trip report template are applied to the trip report, and appear in the Questions view. For performance reasons, the SmartScript questionnaire does not appear in the Questions view until after the user launches the SmartScript questionnaire. For more information about SmartScript questionnaires, see Completing Questionnaires for Clinical Trip Reports .
To apply a clinical trip report template
Navigate to the Site Visits screen, then the Clinical Site Visits List view.
In the Clinical Site Visits list, drill down on the Visit Start field of the site visit to which you want to apply a trip report template.
The Trip Report form for the selected site visit appears.
Click the select button in the Template field.
A list of templates that correspond to the protocol, region, and visit type details for the site appears.
Select the name of the trip report template that you want to apply, and click OK.
When you save the Template field, the activities in the template appear in the Checklist Activities list and Follow-Up Items list.
After the site visit, you record the trip report details, such as:
The planned activities that you complete
Additional activities that you complete
Site personnel that you meet
Any follow-up items that arise from the trip
Comments on any of the previous items
Anyone can update and edit the records in the Trip Report Detail view at any time. For this reason, the end user can create a static report at the completion of the site visit, using the Siebel Report Viewer. This read-only document is ideal for archiving: as a printed document, as a file, or as an attachment to the site record in the Siebel Life Sciences database.
To complete a clinical trip report
In the Clinical Site Visits list, drill down on the Visit Start field of the site visit for which you want to complete a trip report.
On the Trip Report form, complete or edit the fields as shown in the following table.
Navigate to the Checklist Activities view, complete the Status and Comments fields for planned activities, and add any unplanned activities that you completed.
Navigate to the Follow-Up Items view, and complete the following steps:
Add any follow-up activities resulting from the site visit.
In the Current Trip Follow-Up Items list, click Menu (the cogwheel icon), and choose Select All to display all open follow-up items and those items closed between the current and previous trip.
Update the records for those follow-up items that you addressed during the site visit.
Some fields are described in the following table.
Set the Trip Report Status field to Submitted.
The report is submitted to the reviewer for review.
This topic describes how to complete a questionnaire for a clinical trip report. You launch the questionnaire in the SmartScript player from the Questions view of the trip report. Questions and responses along with comments (if any) are saved in the Questions list.
To complete a questionnaire for a clinical trip report
In the Clinical Site Visits list, drill down on the Visit Start field of the site visit for the required trip report.
Navigate to the Questions view.
Click Answer to launch the questionnaire for the trip report in the SmartScript player.
Complete the questionnaire by adding responses and comments (if any).
Click Finish or Finish Later.
The responses and comments are saved with the questions in the Questions list. You can filter questions as follows:
To display only answered questions, select Show Answered.
To display all questions in the questionnaire for the trip report, select Show All.
This topic describes how to delete unanswered questions from a questionnaire for a clinical trip report, using the LS Clinical Questions Batch Clean-up repository workflow process.
To delete unanswered questions from a questionnaire of a clinical trip report
Navigate to the Administration - Server Management screen, then the Jobs view.
Create a new record and complete the necessary fields.
Set the Component/Job field to Workflow Process Manager.
In the Job Parameters list, create a new record and complete the necessary fields.
Set the Name field to Workflow Process Name and the Value field to LS Clinical Questions Batch Clean-up.
Select the record in the Jobs list, and click Submit Job.
Refresh the screen and verify that the State field updates to Success.
All unanswered questions in the questionnaire for the clinical trip report are now deleted.
This topic describes how to create a CRF (case report form) tracking activity. You capture relevant information, such as review date and page numbers verified, for each CRF record.
To track case report forms
In the Clinical Site Visits list, drill down on the Visit Start field of the site visit for which you want to track case report forms.
Navigate to the Case Report Forms Tracking view.
To add case report forms for scheduled subject visits, complete the following steps:
Click Add Scheduled.
Select the subject visits in the dialog box that appears.
The case report forms for selected subject visits appear in the Case Report Forms Tracking view.
To add case report forms for unscheduled subject visits, complete the following steps:
Click Add Unscheduled.
In the case report forms, complete the necessary fields as shown in the following table.
The state model for the trip report provides the order of transition for values in the Trip Report Status field to indicate the progress of the trip report. The following table describes the values for the Trip Report Status field, the update type (such as automatic or user), the trip report validation, and the automated notification emails sent to the assignee’s Universal Inbox for each status, where applicable.
This task describes how to track the real-time progress for a trip report in the Summary view. You track the status for the following fields in the clinical trip report:
Checklists Completed
Questions Answered
Current Follow-Ups Completed
All Follow-Ups Completed
CRF Tracking Completed
Total Attendees
To track the completion status for a clinical trip report
In the Clinical Site Visits list, drill down on the Visit Start field of the site visit for which you want to track the status of the trip report.
Navigate to the Summary view and review the summary status.
This task describes how to track the progress of subject status accruals for a clinical site by creating a real-time snapshot of the current subject status accruals for the site. The CRA (clinical research associate) can use the snapshot to verify the subject status data that an end user manually records during a site visit.
To track status accruals for clinical subjects of a site
In the Clinical Site Visits list, drill down on the Visit Start field of the site visit for which you want to track the status accruals for subjects.
Navigate to the Summary view.
Click Capture in the Subject Status Snapshot applet to generate a real-time snapshot of the status accruals for clinical subjects.
The Inbox provides a centralized list of items requiring your attention, such as clinical trip reports requiring review, revision, and approval. The following procedure shows you how to access and view Universal Inbox notifications for action items of clinical trip reports. Alternatively, you can click Notification on the application banner to access and view all notification messages, including those for clinical trip reports.
To view Universal Inbox notifications for action items of a clinical trip report
From the application-level menu, choose Navigate, then Site Map.
Click Inbox.
The views available for the inbox appear.
Click Inbox Items List.
The list of notifications for your trip reports appears. The action required for each trip report appears in the Name field.
Drill down on the Name field to view each trip report requiring action.
The user designated in the Reviewer field of the Trip Report form can review trip reports with a status of Submitted. An automated notification email is sent to the reviewer when an end user updates the trip report status to Submitted.
Access control applies to the Reviewer Comments field. Only the user designated in the Reviewer field of the Trip Report form can edit the Reviewer Comments field.
To review a clinical trip report
In the Clinical Site Visits list, select My Team’s Site Visits in the visibility filter.
All site visits for your team appear.
Query the list for site visits with a Visit Status field value of Submitted or Submitted for Approval.
For the required report, drill down on the Visit Start field.
The Trip Report form appears.
Review the report.
Set the Trip Report Status field to one of the following values:
Reviewed with Comments. Use this value if minor changes to the trip report are required. Enter the required corrections in the Reviewer Comments field.
Rejected. Use this value if major changes to the trip report are required. Enter the required corrections in the Reviewer Comments field.
Submitted for Approval. Use this value if the trip report does not require additional changes, and is ready for approver review.
Obsolete. Use this value to retire the current or active version of a trip report. This makes it possible to reopen and create a new version of the trip report.
The date and time of the review of the clinical trip report are recorded in the Reviewed Date field.
The assigned approver can approve trip reports with a status of Submitted for Approval. Only the user designated in the Approver field of the trip report has approval permission. An automated notification email is sent to the approver when an end user updates the trip report status to Submitted for Approval. If the approver sets the Trip Report Status field to Rejected, then the CRA (clinical research associate) has the opportunity to revise the report and then resubmit it for approval.
Access control applies to the Approver Comments field. Only the user designated in the Approver field of the Trip Report form can edit the Approver Comments field.
To approve a clinical trip report
(Optional) Add approval comments in the Approver Comments field.
Click Approve.
The User Authentication view appears.
Enter user credentials, and click Verify.
The Trip Report Status field is updated to Approved, the approval date and time are recorded in the Approved Date field, and the site visit record and trip report become read-only.
Active trip reports can be made obsolete at any time.
To make a clinical trip report obsolete
In the Clinical Site Visits list, drill down on the Visit Start field of the site visit that you want to make obsolete.
Set the value in the Trip Report Status field to Obsolete.
The current version of the trip report is retired and the Version button on the trip report is activated. This makes it possible to reopen and create a new version of the trip report.
After a trip report is approved or made obsolete, it is locked (becomes read-only) and the Version button on the trip report is activated. This allows you to reopen and create a new version of the trip report. You can modify and route the new version of the trip through the approvals process again.
You cannot create new versions of trip reports that are active. A message similar to the following appears if you try to create a new version from an active trip report:
Another version of this trip report exists. Use the other trip report version, or change the status of that version to Obsolete and create a new version.
To create a new version of a clinical trip report
Query the list for site visits with a Visit Status field value of Approved or Obsolete.
(Optional) Add reviewer comments in the Reviewer Comments field.
Click Version.
The value in the Version field for the new trip report changes by incrementing by one.
The value in the Trip Report Status field for the new trip report changes to In Progress.
The value in the Trip Report Status field for the original trip report (for approved trip reports only) changes from Approved to Obsolete.
Modify and approve the trip report as required.
For mor e information, see Completing Clinical Trip Reports and Approving Clinical Trip Reports .
The Versions view shows all the prior trip report versions for a selected trip report.
To view version information for clinical trip reports
In the Clinical Site Visits list, drill down on the Visit Start field of the site visit for which you want to view trip report versions.
Navigate to the Versions view.
A third-party application records dates, times, and geographical location details for sites in the trip report for each site monitor visit to each clinical site.
Multiple site monitors can create multiple site visit records in the trip report for the same site visit. Each site monitor can create multiple site visit records in the trip report for different times on the same site visit.
To view geographical location details for a clinical trip report
In the Clinical Site Visits list, drill down on the Visit Start field of the site visit for which you want to view geographical location details.
Navigate to the Geo Location Details view.
The Approvals view provides a summary audit trail of the changes to the trip report status, including the dates and times of review and approval operations, and the details applicable to the users who complete those operations.
To use audit trail for reviews and approvals of a clinical trip report
Navigate to the Approvals view.
The Audit Trail view provides a detailed history of the changes to trip report records. The audit trail records for the trip report show operations for the following fields:
Reviewer Comments
Approver Comments
Trip Report Status
To use audit trail for changes to a clinical trip report
Navigate to the Audit Trail view.
You can integrate Siebel Clinical with Oracle Business Intelligence Publisher (BI Publisher) to generate reports. You can generate, view, and schedule preconfigured Oracle BI Publisher reports in Siebel Clinical. For more information about using Siebel Reports and integrating with Oracle BI Publisher, see Siebel Reports Guide .
The following preconfigured reports apply to site visits:
Clinical Trip Report With CRF
Clinical Trip Report Without CRF
To generate an Oracle BI Publisher report for a site visit
In the Clinical Site Visits list, drill down on the Visit Start field of the site visit for which you want to generate an Oracle BI Publisher report.
On the application toolbar, click Reports.
In the Run Report pane, complete the necessary fields as shown in the following table.
Click Submit.
The report runs.
Click My Reports to navigate to the Reports view of the BI Publisher Reports screen.
A record for the report appears in the Reports view. For information about viewing and printing the report, see Siebel Fundamentals Guide .
Study record managers: refer to the Data Element Definitions if submitting registration or results information.
Search for terms

- Advanced Search
- See Studies by Topic
- See Studies on Map
- How to Search
- How to Use Search Results
- How to Find Results of Studies
- How to Read a Study Record

- Learn About Studies
- Other Sites About Studies
- Glossary of Common Site Terms

- Submit Studies to ClinicalTrials.gov PRS
- Why Should I Register and Submit Results?
- FDAAA 801 and the Final Rule
- How to Apply for a PRS Account
- How to Register Your Study
- How to Edit Your Study Record
- How to Submit Your Results
- Frequently Asked Questions
- Support Materials
- Training Materials

- Selected Publications
- Clinical Alerts and Advisories
- Trends, Charts, and Maps
- Downloading Content for Analysis

- ClinicalTrials.gov Background
- About the Results Database
- History, Policies, and Laws
- ClinicalTrials.gov Modernization
- Media/Press Resources
- Linking to This Site
- Terms and Conditions
ClinicalTrials.gov is a database of privately and publicly funded clinical studies conducted around the world.
Explore 497,586 research studies in all 50 states and in 222 countries..
ClinicalTrials.gov is a resource provided by the U.S. National Library of Medicine.
IMPORTANT : Listing a study does not mean it has been evaluated by the U.S. Federal Government. Read our disclaimer for details.
Before participating in a study, talk to your health care provider and learn about the risks and potential benefits .
- Studies by Topic
- Studies on Map
Patients and Families
Researchers, study record managers.
- Customer Support
- Accessibility
- Viewers and Players
- Freedom of Information Act
- HHS Vulnerability Disclosure
- U.S. National Library of Medicine
- U.S. National Institutes of Health
- U.S. Department of Health and Human Services
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- Current issue
- For authors
- New editors
- BMJ Journals More You are viewing from: Google Indexer
You are here
- Volume 56, Issue 12
- Writing up your clinical trial report for a scientific journal: the REPORT trial guide for effective and transparent research reporting without spin
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- http://orcid.org/0000-0001-6884-1971 Thomas Bandholm 1 , 2 , 3 , 4 ,
- http://orcid.org/0000-0001-9102-4515 Kristian Thorborg 2 , 4 , 5 ,
- http://orcid.org/0000-0001-8102-3631 Clare L Ardern 6 , 7 , 8 ,
- Robin Christensen 9 , 10 ,
- http://orcid.org/0000-0003-1091-2962 Marius Henriksen 9
- 1 Department of Clinical Research , Copenhagen University Hospital, Amager and Hvidovre , Copenhagen , Denmark
- 2 Department of Occupational and Physical Therapy, Physical Medicine & Rehabilitation Research – Copenhagen (PMR-C) , Copenhagen University Hospital, Amager and Hvidovre , Copenhagen , Denmark
- 3 Department of Orthopaedic Surgery , Copenhagen University Hospital, Amager and Hvidovre , Copenhagen , Denmark
- 4 Department of Clinical Medicine , University of Copenhagen , Copenhagen , Denmark
- 5 Department of Orthopaedic Surgery, Sports Orthopedic Research Center – Copenhagen (SORC-C), Amager-Hvidovre Hospital, Faculty of Health Sciences , Copenhagen University , Copenhagen , Denmark
- 6 Musculoskeletal & Sports Injury Epidemiology Centre, Department of Health Promotion Science , Sophiahemmet University , Stockholm , Sweden
- 7 Sport and Exercise Medicine Research Centre , La Trobe University , Melbourne , Victoria , Australia
- 8 Department of Family Practice , University of British Columbia , Vancouver , British Columbia , Canada
- 9 The Parker Institute, Section for Biostatistics and Evidence-Based Research , Copenhagen University Hospital Bispebjerg Frederiksberg , Copenhagen , Denmark
- 10 Department of Clinical Research, Research Unit of Rheumatology , University of Southern Denmark, Odense University Hospital , Odense , Denmark
- Correspondence to Dr Thomas Bandholm, Dept of Clinical Research, Copenhagen University Hospital, Amager and Hvidovre, Hvidovre DK-2650, Denmark; thomas.quaade.bandholm{at}regionh.dk

The REPORT guide is a ‘How to’ guide to help you report your clinical research in an effective and transparent way. It is intended to supplement established first choice reporting tools, such as Consolidated Standards of Reporting Trials (CONSORT), by adding tacit knowledge (ie, learnt, informal or implicit knowledge) about reporting topics that we have struggled with as authors or see others struggle with as journal reviewers or editors. We focus on the randomised controlled trial, but the guide also applies to other study designs. Topics included in the REPORT guide cover reporting checklists, trial report structure, choice of title, writing style, trial registry and reporting consistency, spin or reporting bias, transparent data presentation (figures), open access considerations, data sharing and more. Preprint (open access): https://doi.org/10.31219/osf.io/qsxdz .
- sports medicine
- randomized controlled trial
This is an open access article distributed in accordance with the Creative Commons Attribution 4.0 Unported (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https://creativecommons.org/licenses/by/4.0/ .
https://doi.org/10.1136/bjsports-2021-105058
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
Introduction
You worked hard as the primary investigator of a clinical research project. You spent months preparing the project, 1 and perhaps years collecting and analysing data. You are now ready to report the work as a scientific paper (hereafter ‘trial report’), and submit it to a peer-reviewed, academic journal. You aim for quality and transparency because you want the end-user to be able to read-and-implement for clinical work or read-and-replicate for research. Your coauthors have different and contrasting input to your manuscript draft. How do you navigate this scenario?
Let us introduce the REPORT guide. It is intended to improve reporting of clinical research in general. 2 It is not intended to replace established reporting checklists such as Consolidated Standards of Reporting Trials (CONSORT) 3 —they are always your ‘first choice’ reporting guidance resources. Rather, we intend the REPORT guide as a ‘How to’ implementation guide and directory that holds tacit knowledge (ie, learnt, informal or implicit knowledge) and references to sources of information about effective and transparent trial reporting. We have included information on topics we have struggled with ourselves as authors and see authors struggle with when we review or edit submitted clinical trial research. We published the PREPARE trial guide in 2017 1 which aimed to assist in the preparation and planning of clinical trial research. The REPORT guide is a natural extension of PREPARE—focusing on reporting of clinical trial research. If you used the PREPARE trial guide 1 to plan your research, the REPORT guide will likely help you report it. The REPORT guide can also function as a stand-alone guide to help you report research no matter how it was prepared.
The REPORT guide provides information to help improve reporting quality and transparency. The focus is the randomised controlled trial (RCT) (hereafter ‘trial’), but the guide is useful for other study designs.
The CONSORT checklist and CONSORT-based web tool writing aid tool: an important first step
An important first reporting step is to locate a reporting checklist that matches your study design. A comprehensive list of reporting checklists can be found at the EQUATOR network’s website. 4 For a trial, the appropriate reporting checklist is the CONSORT checklist 3 for which there are several extensions that may be relevant. We encourage you to go to the ‘Toolkits’ section at the EQUATOR network’s website 5 where you can find information to help you select the appropriate reporting checklist. You may also find the CONSORT-based WEB tool (COBWEB) 6 useful in your writing and checklist adherence. As stated on the COBWEB(site): ‘COBWEB is an online manuscript writing aid tool intended to guide authors through the process of manuscript writing of RCTs in line with the Consolidated Standards of Reporting Trials (CONSORT) and its subsequent extensions’. We highly recommend you use this tool, as it will facilitate effective trial reporting. It will help you avoid many of the documented problems with CONSORT adherence, such as poor reporting of randomisation methods or description of sample size estimation. 7
For more information on reporting checklists: 1 4 6 8 9
Keep the trial protocol and registration next to you as you write the report
Journal reviewers and editors will be some of the first professional readers of your trial report when submitted to an academic journal. They want to know if you did what you set out to do and—if not—why you made changes. They will look at your trial registration and protocol, if publicly available or submitted with the trial report—comparing the information in the trial registry to that in the trial report and looking for consistency for important trial characteristics. Authors of systematic reviews will do the same when they include your trial—once published—in their review and assess bias, for example, in the selection of your reported results. 10
We encourage you to take the same approach as reviewers and editors when you write your trial report. Have the trial protocol open and the registration available when you write—generally use a copy-paste approach for important trial characteristics to increase transparency and consistency for two related work packages of the same research project ( figure 1 ). Sometimes the trial report will be flagged by plagiarism checkers that journals use because the methods sections are very similar. Ensure you reference previous work and consider presenting the argument for this approach in the cover letter and/or the trial report itself. The guide ‘Avoiding Plagiarism, Self-plagiarism, and Other Questionable Writing Practices: A Guide to Ethical Writing’ by Dr Miguel Roig is a helpful and detailed resource. 11 Roig made the case for more editorial flexibility when it comes to textual reuse of technical descriptions—especially for writers who do not have English as their first language. 12 Finally, check any author/publisher copyright agreement if you have published your trial protocol to avoid any copyright infringement.
- Download figure
- Open in new tab
- Download powerpoint
We encourage you to have the protocol open and trial registration available when you write. If you use a copy-paste approach, it will facilitate consistency between trial protocol, registration and report.
Using a copy-paste approach will help you report important trial items in the same order and with the same wording as used in the registration. Examples of important trial items include aim, selection criteria and outcomes. If you deviated from the plan (common and acceptable with a reasonable explanation) transparently report it and why. Many journals require that you upload the trial protocol as a supplemental file to the trial report. A copy-paste approach creates a strong link between these two documents and increases readability. If wording cannot be copied and pasted 100% for consistency, we suggest you check carefully if the meaning is still the same. For example, you may have come up with a better title after having revised the trial report many times, or you realised that the trial objective could have had better wording.
Writing your trial report
Structure: introduction, methods, results and discussion.
Most scientific journals prefer a trial report style that follows the IMRaD-structure (ie, I ntroduction, M ethods, R esults a nd D iscussion). 13 You can find useful generic information on how to structure scientific papers from the PLOS collection. 14 We provide additional information relevant to the different sections of a trial report below.
Crafting a ‘tempting title’
Declarative and descriptive titles are the typical types of titles a reader is likely to encounter in the sports & exercise medicine field. A declarative title declares the key message (often a key result; eg,’ Meniscus or cartilage injury at the time of anterior cruciate ligament tear is associated with worse prognosis for patient-reported outcome 2–10 years after anterior cruciate ligament injury: a systematic review’). 15 A descriptive title describes what the reader can expect to find in the trial report (often the type of study, the population or the outcome; eg’ Which treatment is most effective for patients with Achilles tendinopathy? A living systematic review with network meta-analysis of 29 RCTs’). 16 When crafting a title for your trial report, consider whether you are aiming to engage the reader, inform the reader, or both, and if possible finalise the title with the design of your study.
Scientific writers can be creative without being frivolous, trivial or unscholarly/unscientific. A tempting title does not mean one has engaged in ‘spin’. 17 We recommend aiming for a declarative title where possible—the title is your chance to share a powerful first impression with your reader—although, we recognise you do not always have a choice. Adopting the engaging: informative style 18 offers a way to let your creativity flow, without straying too far from scholarly conventions and inviting the stroke of an obstinate editor’s correction pen. Here is one example:’ Running themselves into the ground? Incidence, prevalence, and impact of injury and illness in runners preparing for a half or full marathon’. 19
Your tempting title might comprise two, or even three, parts: (1) the hook: perhaps a play on words or a metaphor, (2) the key message, where you declare why the reader will want to read on or what she will find if she reads on, and (3) a key distinguishing feature of your trial report: perhaps a characteristic of the population, the type of trial (eg, double-blind superiority trial) or time frame for data collection). To facilitate correct PubMed indexing and identification, the CONSORT group 3 encourages authors to include the study design in the title (eg, ‘an RCT’).
The stylish academic writer: three suggestions to help you capture and engage your reader’s attention
Scientific writing and creative writing are not polar opposites. Our statement in the last section on tempting titles bears repeating: scientific writers can be creative without being frivolous or unscholarly. Like with your title, we recognise you may not always have a choice about some aspects of style (eg, some journals require third person perspective and forbid using first person pronouns like ‘ we measured quadriceps strength using an isokinetic dynamometer.’).
Suggestion 1: use concrete language and banish passive sentences
Consider replacing ‘There are numerous approaches to the quantification of training load’ with ‘There are at least three tools to measure training load’. Even more concrete is: ‘We describe three tools clinicians could use to measure training load in recreational runners’ because (1) your reader knows how many ways to measure training load she can expect to read about, and (2) she knows something about the population. She also knows who is doing what to whom: clinicians (who) are measuring (doing what) the training load of recreational runners (whom). Numbered or ordered lists help you organise your thoughts and convey a clear message to your reader.
Suggestion 2: write in active sentences that are driven by active vivid verbs
Even when your writing context is constrained or less flexible (or perhaps inflexible) given journal requirements, we encourage you to address your reader directly—with active writing. One can choose to write concise, clear, coherent sentences or one can choose vague, passive, verbose sentences. Which sentence holds your attention as a reader? Concrete language uses active verbs (eg, describe, explore, compete, measure), avoids abstract nouns (eg, quantification, conversation, completion, effectiveness, discretisation) and clarifies who is doing what, to whom.
Suggestion 3: comb your manuscript for be-verbs and replace them with active verbs
Forms of be, including was, were, been, being, are, is or shown, are also juicy targets for writers who are aiming to resurrect their writing. Passive verb constructions like ‘can be measured’ or ‘were shown’ weigh your writing down. Try replacing a few be- verbs in each paragraph with active, vivid verbs (eg, masquerade, prescribe, roll, shun).
Writing well is a deliberate, careful and considered process. It is a craft that requires time and practice. You will find writing resources and suggested reading on renowned Professor of Linguistics and scientific writing coach Helen Sword’s website. 20 Four of the five authors of the REPORT guide do not have English as their first language. In scientific writing, we use the three suggestions above. We also use a ‘how simple can you go’ approach to guard against major linguistic mistakes and to increase readability for readers whose first language is not English. In Lingard et al ’s excellent Writing Craft series, 21 they identify key grammatical challenges and offer practical tips for native Spanish, French, Dutch and German speakers who are writing in English ( table 1 ). 22
- View inline
Key grammatical challenges for Spanish, French, Dutch and German researchers writing in English
Three things to try:
Watch Professor Sword explain how to avoid nominalisations. 23
Run your writing through an online workout. 24
Train with some Wordcraft Workouts . 25
Abstract: the CONSORT-way
The trial report abstract is important because it will likely have many more reads than the full trial report. Most journals have a word limit for abstracts, and some have mandatory structure and headings. Each of these restrictions can pose a challenge when writing a clear, transparent and detailed abstract—you need to make every word count. 21 If journal formatting allows, use the CONSORT for reporting randomised trials in journal and conference abstracts. 26 It comes with an explanation and elaboration paper 27 as well as an abstract item checklist, which can be downloaded from the CONSORT website. 28 Preliminary work from the CONSORT group showed that all the checklist items can fit within 250–300 words. 27 The CONSORT website also has a sample study that implements the CONSORT checklist. 29 The sample study includes an example of how to write abstract results, which can be problematic. 30
When you state the trial framework, for example, ‘superiority trial’ it creates an excellent link to what follows in the abstract. It links to which intervention the authors hypothesise to be superior to the comparator (objective); the main outcome and time frame that this is assessed (primary outcome and endpoint); indication of risk of bias (randomised vs analysed, blinding, trial registration), indication of superiority (effect size, between-group difference in response for the primary outcome) and claim of superiority as hypothesised (conclusion). To avoid unintentional reporting 31 or spin 32 biases in the conclusion, we suggest you reserve the first line to conclude on your objective and corresponding primary outcome and use PICOT 33 as the framework (Population, Intervention, Comparator, Outcome, Time frame). For example, ‘Compared with intervention C (the comparator), the intervention of interest I was not superior in reducing O (primary outcome) at T (time frame) in P (population). We encourage you to then continue with secondary outcomes: ‘For the secondary outcomes X, Y and Z, we found that (…)’ ( figure 2 ).
We encourage you to create a strong link between the conclusion and the trial aim and hypothesis if you think ‘aim’, ‘hypothesis’ and ‘trial design’ when you write the first line of the conclusion.
If your trial was prospectively registered, we suggest you state this at the end 26 of the abstract as ‘Trial identifier: (number) followed by ‘(prospectively registered)’. If for some reason your trial was not registered before the first participant was included (prospectively/preregistered), we suggest you transparently report this at the bottom of the abstract as ‘Trial identifier: (number)’ followed by ‘(retrospectively registered)’. This is currently the editorial policy for all BMC journals when they consider retrospectively registered trials for publication. 34 In the main trial report we encourage you to explain the reason for this practice and state if important trial changes occurred after the trial began, as there will be no publicly available record of your research intentions. If you posted your trial report as an open access preprint, we encourage you to add the preprint information to the bottom of the abstract. In the REPORT guide, you will find an example of this use (more info about preprints below). It will help the reader find an open access version of your trial report and link the two documents via the digital object identifier (DOI) 35 ; ‘Preprint (open access): http://doi.org/ (doi number)’. We suggest you use a copy-paste approach for important abstract information, so that it is consistent with the trial registration and/or published protocol as well as main trial report document (eg, aim and conclusion).
For more information on abstract reporting: 21 27 36
Introduction: the ‘why’ of your trial
In this section, we encourage you to present the ‘why’, that is, an argument for why your trial is needed. If the ‘why’ is not clear to you and your coauthors, it will be difficult to convey it in a trial report. Readers are already motivated because they screened your title and abstract for relevance and results. However, the Introduction helps the journal’s reviewers and editors judge the importance of your trial report. It is therefore essential to making it interesting, while at the same time concise and describe the knowledge gap that your trial is intended to fill.
Your introduction should present the scientific background and rationale. 3 It should follow the background section of your protocol, as the reason for doing the trial has not changed. Thus, the introduction can more or less be copy-pasted from your protocol. During the planning and conduct of your trial, however, others may have published relevant research findings. They may support or oppose your results but should be mentioned. The introduction should include a summary of relevant studies as an up-to-date systematic review or at least include the latest published systematic review on your topic. It is important not to be selective in this literature review as it may mislead the reader, increase the risk of confirmation bias 37 or unintentionally communicate that the knowledge gap is larger or more important than it is. Consider letting the reader know that you have made steps to avoid this by stating that you have scrutinised all available evidence and use the best available evidence to inform the need of the trial: ‘The latest systematic review with meta-analysis on the effectiveness of (your intervention) on (your outcome of interest) concluded that (main finding). This is supported by two recent trial reports published after the systematic review by (author)’.
While the background information in a trial protocol oftentimes is very lengthy, the introduction part of a trial report can be shorter. Consider who will read your trial report and try to direct the introduction to that audience. For specialty journals it is not necessary to state general knowledge in the field. If you write about treatment of sports injuries and intend to publish in a sports medicine journal, it is unnecessary to write elaborately about the prevalence, costs, injury mechanisms or importance of treatment effectiveness. Readers of the journal will know this information already. Focus more on your trial rationale, specific research question, and aim. By cutting to the chase, you will save words that are better spent elsewhere.
End the introduction by stating the aim or the objective of your trial and include the hypothesis. Aims and hypotheses are not always easy to differentiate, but hypotheses are typically more specific and relate closely to the chosen trial design, outcome measures and statistical analysis plan (SAP). This is the most important part of the introduction. We suggest you copy/paste from the trial registry and/or published protocol for consistency ( figure 1 ). We also suggest using the copy/paste approach for abstract and main trial report so that the aim in the abstract is the same as the one in the main trial report.
For more information on systematic reviews to fully use previous research: 38 39
Methods: the ‘how’ of your trial
In this section, we encourage you to present the ‘how’ of your trial. What did you do in order to answer the ‘why’? The methods section is a detailed description of what was done and serves at least two main purposes: (1) to provide enough information to allow the reader to critically appraise and interpret the results, and (2) to convey as many details as possible so other researchers (in principle) will be able to replicate you trial entirely or in part. For clinical application of your trial results, it is important to give detailed descriptions of the population selection, assessment methods, and interventions. Other important aspects of the methods section are central for evaluating the scientific quality, validity and reliability of the trial.
Ideally, the methods section should be a replica of your trial protocol. But completing a trial without ‘bending the rules’ laid out in the protocol is practically impossible. It is therefore important to report any deviations and violations of the protocol. It is not a ‘scientific crime’ to deviate from the protocol, but it is important to report any deviation with potential bearing on your primary and important secondary outcomes (and thus on the interpretation of the entire trial). It is particularly important to declare ‘planned’ deviations, such as changes to eligibility criteria (eg, due to safety or slow recruitment), changes in instruments (eg, change of MRI-scanner due to breakdown). We suggest you report the deviations with reasons and describe what you did.
When you write your trial methods, imagine that your trial report 1 day will be scrutinised as part of a systematic review or clinical guideline. Reviewers will appraise your trial report on the lookout for flaws (or risks of bias). While you may have conducted your trial scrupulously (ie, with a low risk of bias), reporting can be incomplete. Reviewers may be uncertain about aspects of your trial methodology, which may mean they must downgrade the quality of your trial. We suggest you consult The Cochrane Handbook for Systematic Reviews of Interventions. 40 It provides detailed information on how to appraise individual trials, and provides you useful hints on what reviewers are looking for. For example, a reviewer may look for the phrase ‘sequentially numbered, opaque, sealed envelopes’ when assessing risk of bias for ‘Allocation concealment’. Knowing this, will help you clearly report how this was done in your trial.
For more information on how to report protocol deviations and risk of bias assessment: 40 41
Methods: outcomes
The CONSORT checklist items for ‘Outcomes’ ask you to report ‘Completely defined pre-specified primary and secondary outcome measures, including how and when they were assessed’ and ‘Any changes to trial outcomes after the trial commenced, with reasons’. 3 If you use a copy-paste approach, it will be easy to copy from your protocol and preregistered trial summary and paste into the trial report. It will create consistency with regards to, for example, number of outcomes, outcome hierarchy and wording. If for some reason you had to add or remove outcomes during the trial, we recommend you report this transparently, with reasons.
The COMPare trials project 42 team systematically checked every trial published in the top five medical journals between October 2015 and January 2016, with the purpose of searching for misreported findings and outcome switching. This team’s effort revealed a large degree of inconsistency in outcome reporting. 43 44 If for some reason, you had to make changes to your trial outcomes after the trial began, state this transparently and give reasons for the changes. If you plan to report collected outcomes in subsequent (secondary) trial reports, we suggest you state in the primary trial report that the outcomes were collected—consistent with the trial registry—and that you plan to report them in a subsequent report. This could be the case if you collected mechanistic and more exploratory outcomes in your trial, such a blood samples that await future advanced molecular analysis. Stating that these were collected will help you avoid misreporting of outcomes. The COMPare trials project 42 state in their Frequently Asked Questions-section: ‘Question: What if some outcomes are reported in a different publication? Answer: This is fine, as long as this fact has been declared in the trial publication. For example, if a trial says here we are reporting A B and C, in a subsequent paper we will report X Y Z then the outcomes X Y Z are not considered as unreported, and they are removed from the denominator. ’. 45
For more information on how to report trial outcomes: 3 42 46
Methods: interventions
Proper reporting of interventions is especially important for clinical application of your trial interventions, correct interpretation of your trial results, comparison with other trials (with similar interventions) and ability to inform new research questions. Unfortunately, intervention reporting is generally poor. 47 To help you report your interventions, The Better Reporting of Interventions: Template for Intervention Description and Replication checklist was developed. 48 It will help you make a complete and thorough generic description of the interventions. You may also need to consult a more intervention-specific guide or reporting checklist, such as the Consensus on Exercise Reporting Template for exercise trials. 49 We suggest you describe any ‘usual care’ or other comparator intervention using the same standards and checklists. For some comparators, reporting checklists have been developed—one was just developed for placebo and sham controls. 50 You may already have published a detailed description of your intervention and comparator as part of a published protocol. In case changes were made to the intervention or its delivery during the trial, consider if the description needs to be updated and submitted with the trial report as supplemental material. It will help both replication and clinical implementation of your trial results. In case you have not already published a detailed intervention description, consider publishing it as supplemental material to your trial report. It will help you if the journal has a word limit for the main document.
For more information on how to report trial interventions: 48 49
Methods: sample size
This sample size paragraph is intended to outline how you, in the trial planning phase, ensured that the trial would have the required statistical power to identify whether a difference of a particular magnitude (the target difference) exists for the primary outcome. It is also intended to show that you did not include any extra participants than were needed for the trial. As you did all the thinking already, it should be feasible to copy/paste from the trial protocol. The basics of calculating sample size are covered in substantial detail in the PREPARE Trial guide. 1
For more information on how to determine and report the target difference and sample size estimation for a trial: 51
Methods: statistical analyses
The problem of poor statistical reporting is long-standing, widespread, potentially serious and yet is largely unsuspected by many readers of the biomedical literature. 52 General guidance on how to write SAPs is now available 53 and provides recommendations for a minimum set of items that should be addressed for clinical trials before analysing data. 53 If you have not written a specific SAP as part of the trial protocol, 1 we recommend that you consult a biostatistician and write one before viewing any data or starting the analysis.
An SAP ensures that the statistical methods are reported in sufficient detail to enable a knowledgeable reader (with potential access to the original data) to assess the appropriateness of the chosen statistical methods and the underlying assumptions, and to verify your reported results. In a SAP, the statistical methods are often described in great detail, and a complete copy-paste approach may be too much (given that most journals have restrictions on manuscript length). We therefore recommend always submitting the SAP (with final date on cover page) as supplemental material so that editors, peer reviewers and other readers can take a deeper dive into the statistical methods.
In the main text, we encourage you to give an extract of the primary statistical analyses (from the SAP). If you have stated a clear objective, the reader will be able to understand the primary purpose of the trial and what to expect to see next. We recommend that you describe fully the main methods for analysing the primary and key secondary objectives of the trial. It is common to analyse the data set under different assumptions—sensitivity analyses—to assess the robustness of the primary analyses. These are typically based on different strategies for handling missing data or analyses of different trial populations (eg, the per protocol population which is potentially biased but still informative). We recommend you carefully describe these strategies. Excellent educational resources exist to assist you. They include the CONSORT explanation and elaboration paper, 3 the SAMPL guidelines for statistical reporting, 54 and the recently developed Checklist for statistical Assessment of Medical Papers statement. 55
For more information on how to report statistical analyses for a trial: 3 54 55
Results: attrition
Attrition can introduce bias in your trial results if the characteristics of participants who are lost to follow-up differ between the randomised groups—especially if the differing characteristic is related to trial outcome measures. 56 If you use the CONSORT flow diagram to illustrate the trial profile, we suggest you report the demographics of the participants included in the intention-to-treat population with descriptive statistics for each group. We encourage you to create an overview by preparing a classic table 1 of baseline characteristics using the outline from the CONSORT explanation and elaboration paper. 3 You may also find it useful to supplement with table items as suggested by Dumville et al 56 and attach as a baseline appendix.
Reviewers will sometimes ask for results of statistical testing for baseline differences. The recommendation from the CONSORT group is clear: ‘Such hypothesis testing is superfluous and can mislead investigators and their readers. Rather, comparisons at baseline should be based on consideration of the prognostic strength of the variables measured and the size of any chance imbalances that have occurred’. 3 It means you should subjectively judge if any differences between groups that will occur by chance due to randomisation is of a magnitude that you think is clinically relevant.
For more information on how to report attrition: 56
Results: focus on the main analysis and between-group differences
Correct reporting of the results of the statistical analyses includes explicit estimates presented with appropriate indicators of measurement error or uncertainty, such as 95% CIs. Randomised trials are designed to analyse differences between groups, and the results should focus on these—not on changes within groups. However, it is helpful for transparent reporting and interpretation to present the estimates in each group. We strongly recommend that you avoid reporting only statistical hypothesis testing (eg, such as P values), as they do not contain much information and do not convey important information about effect sizes or precision of estimates. When you report p values, we recommend you report actual p values, rather than p<0.05, unless the value is very small (eg, p<0.0001).
We suggest you report your primary analyses first and hierarchically (primary outcome before secondary and other outcomes). This will most likely follow the hierarchy outlined in your trial protocol and SAP. By being consistent and use a copy/paste-approach, you will help the reader assess if you followed your SAP. We recommend you avoid interpretations or interpretative language in the results section, but instead help the reader by providing a direction of the results and whether it favours one of the groups. In cases where discrepancies between analysis sets occur among the primary analyses and the sensitivity analyses, we suggest you highlight them in the text. You may also need to devote more attention to interpreting the collective results because the confidence in the individual analyses is reduced.
During your data analyses, new and exciting ideas may arise, as well as unexpected findings that you did not consider during planning. Such results can be important and foster significant scientific advances. However, consider that your trial design may not support confirmatory analyses or statements of such findings, and it is important to state (both in the Statistical methods section and in the Results section) that these were not prespecified. Related to this topic is the situation where a peer reviewer asks for additional analyses of your data set; that is, frequently referred to as the ‘peer review pressure test’. These are often valuable and reasonable requests, but should very rarely replace the original analytical strategies, unless there are fundamental flaws in the trial design and/or the chosen analyses do not reflect the experimental design. We suggest that post hoc analyses requested by peer reviewers are reported in supplemental files and included in a response letter to the reviewers when submitting a revised manuscript.
For more information on how to report the results of statistical analyses for a trial: 54 55
Results: transparent illustration of your data
Your tables and figures should ideally be able to stand alone (eg, in presentations and lectures). It is valuable to provide brief summaries of the statistical methods used (eg, as foot notes to tables and figures). The CONSORT checklist and explanatory paper have great examples and descriptions of how to make certain illustrations. On the CONSORT website, you will find a flow diagram template freely available for download. 28 We suggest you add some additional information to the flow diagram, including numbers of participants included in the different analyses (eg, intention-to-treat and/or available case analysis) and number of imputations made for missing data, if applicable. For an example, please see Lysdal et al 57
Include specific information on your sampling strategy at the top of the flow diagram because it will facilitate interpretation of the trial findings with regards to clinical relevance. Consider reporting the total number of potentially eligible participants during the trial recruitment period and how many of these were assessed for eligibility, instead of only reporting the number of individuals assessed for eligibility. It allows the reader to judge how well the trial population represents all patients seen at the recruitment site while the trial was running. Because this issue relates to external validity it is important—but it is especially important if the trial findings have major implications for current clinical practice. Please see Clausen et al 58 for an example of how the number of potentially eligible participants can be incorporated into the trial flow chart. Please also see the rapid responses 59 to the FIMPACT 60 trial for a discussion of the importance of including the number of potentially eligible participants when trial findings have great implications for clinical practice.
Results may sometimes merit a figure in the form of a graph. Many bar or lines graphs—based on continuous data with different distributions—can lead to the same bar or line graph ( figure 3 ). 61 Unless you include raw data in the graph, most information will be invisible to the reader. We encourage you to making it visible by using scatter plots instead of bar charts.
Many different datasets can produce the same bar graph. The figure and legend are modified from Weissgerber et al 61 https://doi.org/10.1371/journal.pbio.1002128 under the terms of the creative commons CC by 4.0 license https://creativecommonsorg/licenses/by/40/ .
For more information on how to effectively use tables and figures in scientific papers: 61–63
Results: harms
When new healthcare interventions are studied, researchers tend to focus more on efficacy than safety. There is poor reporting of harms in trial reports across many clinical areas, 64 which makes it difficult to obtain a true estimate of the benefit-harms ratio. The CONSORT extension for harms 65 was developed to improve reporting of harms-related data in trials. Because the main focus of the CONSORT checklist is efficacy reporting, we suggest you supplement your trial reporting with the CONSORT extension for harms 65 to improve reporting of harms-related data.
For more information on reporting of harms-related data: 65

Discussion: consider clinical relevance and confirmation bias
The CONSORT checklist 46 holds the overall framework for the discussion and items you should address, but scientific journals may have additional requirements. We suggest you use the CONSORT checklist to structure the discussion, and supplement with requirements from your target journal, if needed. We would like to highlight two important items: clinical relevance and confirmation bias.
We suggest you focus on the primary analysis and outcome. Your trial was designed first and foremost to provide a reliable answer in terms of the hypothesis for this analysis and outcome. The test statistics will determine if the difference between groups is statistically significant. Judging and discussing whether a statistically significant difference between groups is also clinically relevant should be easy at this point. You will already have argued in your trial protocol and sample size paragraph what minimum theoretical difference between groups you consider clinically relevant and why. Now that you have the observed difference between groups, the main issue is to compare the two and discuss the size of the observed effect. An important aspect of this discussion is the precision of the observed effect. In general, the larger the sample size of your trial, the greater the precision of the observed effect. The precision is reflected in the 95% CI of the observed effect. The greater the precision, the smaller the 95% CI and vice versa. We suggest a balanced discussion of the clinical relevance of the observed effect to include both its size (in relation to the predefined minimal clinically importance difference) as well its precision. It will help you avoid unintentional confirmation bias (please see below).
Biases come in many forms and can affect healthcare in many ways. There may be biases that you want to acknowledge specifically under ‘Limitations’ in the discussion because you think they may have influenced trial procedures or outcomes. We suggest you consider your own ‘confirmation bias’ when writing the discussion—or the whole trial report for that matter. As stated by the Catalogue of Bias Collaboration 37 : ‘Confirmation bias occurs when an individual looks for and uses the information to support their own ideas or beliefs. It also means that information not supporting their ideas or beliefs is disregarded.’ Being researchers, we argue that most of us unintentionally wish for our intervention to be superior to the comparator for several reasons: (1) we want to help patients by advancing the field, or (2) we think it will bring promotion or other academic reward. By being intentionally aware of our own confirmation bias, we can better stay clear of issues such as unintentional secondary analysis emphasis (spin) and selective referencing of work that support our own findings.
For more information on statistical significance, clinical relevance, spin and confirmation bias: 17 37 66
Conclusion: what was your trial designed to test first and foremost?
When you write the trial report conclusion, we encourage you to think ‘aim’, ‘hypothesis’ and ‘trial design’. What was your trial designed to test primarily and how was this formulated in the aim? Was it to assess if the intervention of interest was better than (superiority trial), no worse than (non-inferiority trial), or whether it was as equally effective as (equivalence trial) the comparator? Using this line of thinking will help create a strong connection between aim, hypothesis and conclusion. It will also help you conclude only what the trial data support. If the aim of a superiority trial was ‘To investigate if I (Intervention of interest) is superior to C (comparator) in improving O (primary outcome) at T (timepoint) in P (population) and there was no difference in response between groups, the conclusion could start with: ‘Compared with C (comparator), I (Intervention of interest) was not superior in reducing O (primary outcome) at T (time point) in P (population). A very common mistake is to interpret the absence of evidence of superiority as evidence of equivalence or non-inferiority and conclude that the intervention of interest and comparator were equally effective or no different (for more details, please see refs 1 67 ).
Having addressed the main hypothesis, analysis and outcome the trial was designed to assess, we encourage you to proceed with interesting secondary analyses and—at the same time—inform the reader about the increase in risk of bias for these analyses: ‘For the secondary outcomes, X, Y and Z, we found that (………).’. When you conclude first on the primary analysis, you minimise the risk of unintentional reporting 31 or spin 32 biases. If your trial was more exploratory than confirmatory 1 —or had a flat outcome hierarchy with no single primary outcome—you may want to consider finishing the conclusion by acknowledging this. For example, ‘This finding needs replication in future trials’. Readers will often be interested in your thoughts on the implications of your trial findings. Some journals allow implication statements and others do not. If you do write about implications, we suggest you make it clear that this part of the conclusion is you speculating and conveying your expert opinion with phrasing like: ‘These findings may have implications for (……) insofar as (……).’. When you have finished writing the conclusion, check that it matches the trial aim and conclusion in the abstract.
Sharing research data
Depending on national legislation, you may or may not be able to share the raw trial data. Data sharing is one way of increasing transparency and maximising the trial participants’ research contribution by making the data they provided broadly available for secondary research purposes. 68 69 Data sharing is also expected by some non-private funding bodies. 70 If you can share your trial data, there are some things that you may want to consider. They include practical steps to data management, anonymisation and storage.
For more information on data sharing: 71–74
Alternative avenues for disseminating your research
When you are ready to submit your trial report to a scientific journal, consider publishing a preprint. A preprint is scientific work that has not undergone peer review and is not published in a scientific journal. 75 It is typically a manuscript draft that is ready to be submitted to a journal for peer review. A preprint can also be an earlier manuscript version that you want to make public. One advantage of publishing a preprint is that it is assigned a DOI, 35 which makes it searchable. Most publishers allow preprints, 76 but we suggest you check the preprint policy of the scientific journal that you aim to submit your trial report to. Elsevier states: ‘Preprint: Authors can share their preprint anywhere at any time. If accepted for publication, we encourage authors to link from the preprint to their formal publication via its Digital Object Identifier (DOI). Millions of researchers have access to the formal publications on ScienceDirect, and so links will help your users to find, access, cite, and use the best available version. Authors can update their preprints on arXiv or RePEc with their accepted manuscript. Please note: Some society-owned titles and journals that operate double-blind peer review have different preprint policies . ’. 77
Submission to preprint servers is typically free and it creates an open access option, even if you end up publishing your trial report behind a paywall. 78 It allows you to have crowdsourced feedback and to promote your open access research early (eg, during the period of peer review). Based on feedback, you can update your preprint version when you revise your manuscript. Some (but not all) publishers even allow you to update your preprint to the accepted (non-type set) manuscript version with proper reference to the journal publication. Please check the publisher’s preprint policy for guidance. If you look at the bottom of the abstract of this guide, you will see a link to an open access preprint. Had this guide not been published open access, an interested reader could see from the PubMed abstract where to find an open access full text (preprint).
For more information on preprints: 75
Researchers are familiar with social media platforms like Twitter for sharing new scientific content. When posting to social media, make space to include (1) the DOI 35 and (2) an image—two simple steps to help make your post visible to attention metrics-aggregators like Altmetric and capture the viewer who might otherwise scroll past your post. Across research areas, the Altmetric score has been associated with number of citations, journal impact factor, press releases and open access status. 79 80
Have you considered other forms of media? Researchers who embrace the rich ecosystem of digital media might find themselves partnering with clever infographics designers or using free (or freemium) websites to design their own. Consider writing for trusted outlets like The Conversation 81 —a news organisation that is dedicated to sharing information from the academic and research community, direct to the public, with ‘academic rigour and journalistic flair’. Sports medicine and sports physiotherapy journals including British Journal of Sports Medicine and Journal of Orthopaedic & Sports Physical Therapy have blogs dedicated to reaching a non-academic audience of clinicians, patients, athletes and coaches.
Consider approaching your academic institution’s media and communications department or press office. The staff are typically pleased to work with you to shape a press release, distribute the press release to mainstream media services, and connect with media contacts. Media and communications departments also share helpful tips for making your research visible to the media. 82
After publishing the trial report
After your trial report is published, consider (1) Is the ‘Trial status’ up to date in the trial registry? (2) Do I need to update the trial registry with a link to the published trial report and/or raw data if shared? (3) Do I need to report to funding bodies on the accomplished milestone (publication)? (4) Do I have a plan for disseminating the trial results other than the primary trial report? (5) Do I have a plan for storing and filing essential trial documents and data that adheres to national guidelines?
We hope the REPORT guide is helpful and a valuable supplement to ‘first choice’ trial reporting tools, such as CONSORT. We aimed to incorporate tacit knowledge about reporting, and flag issues we have struggled with. Quality decisions in healthcare depend on reliable evidence of treatment effects. Good research reporting practice does not cure ‘diseases’ that arise from poor research methodology—it helps the reader see the illness and appraise the research quality. No research is perfect. We do not profess to produce and report perfect research that is free from ‘disease’ 100% of the time. We implore all researchers to commit to conducting (and reporting) clear and transparent research.
What is already known
Reporting of clinical trial research varies and is known to be poor.
What are the new findings
The REPORT Trial guide is a one-stop, ‘how-to’ implementation guide and directory that holds tacit knowledge and references to first-choice sources of information about effective and transparent trial reporting (eg, CONSORT).
Ethics statements
Patient consent for publication.
Not applicable.
Ethics approval
This study does not involve human participants.
- Bandholm T ,
- Christensen R ,
- Thorborg K , et al
- Glasziou P ,
- Altman DG ,
- Bossuyt P , et al
- Hopewell S ,
- Schulz KF , et al
- The EQUATOR Network
- ↵ Toolkits | the EQUATOR network . Available: https://www.equator-network.org/toolkits/ [Accessed 1 Dec 2021 ].
- ↵ COBWEB Consort-Based web tool: an online writing aid tool for writing a randomized trial report . Available: https://cobweb.clinicalepidemio.fr/ [Accessed 1 Dec 2021 ].
- Shams I , et al
- Shanahan DR ,
- Lopes de Sousa I ,
- Marshall DM
- Bliddal H ,
- Henriksen M
- Sterne JAC ,
- Savović J ,
- Page MJ , et al
- Sollaci LB ,
- Pedersen M ,
- Johnson JL ,
- Grindem H , et al
- van der Vlist AC ,
- Winters M ,
- Weir A , et al
- Franke TPC ,
- Backx FJG ,
- Huisstede BMA
- ↵ Helen sword | writing with Pleasure. Helen sword . Available: https://www.helensword.com [Accessed 1 Dec 2021 ].
- Lingard L ,
- Cristancho S ,
- Hennel EK , et al
- ↵ Beware of nominalizations (AKA zombie nouns) - Helen Sword , 2012 . Available: https://www.youtube.com/watch?v=dNlkHtMgcPQ [Accessed 1 Dec 2021 ].
- ↵ The Writer’s Diet . Available: http://writersdiet.com/ [Accessed 1 Dec 2021 ].
- Online writing program
- Moher D , et al
- ↵ CONSORT - Welcome to the CONSORT Website . Available: http://www.consort-statement.org/ [Accessed 1 Dec 2021 ].
- ↵ CONSORT - Example Documents and Sample Studies . Available: http://www.consort-statement.org/examples [Accessed 1 Dec 2021 ].
- Krummenauer F ,
- Geis B , et al
- Mahtani KR ,
- Chalmers I ,
- Thabane L ,
- Ye C , et al
- ↵ Retrospectively registered trials: the Editors’ dilemma. Research in Progress Blog , 2016 . Available: https://blogs.biomedcentral.com/bmcblog/2016/04/15/retrospectively-registered-trials-editors-dilemma/ [Accessed 1 Dec 2021 ].
- Spencer EA ,
- Evidence-Based Research Network
- Brunnhuber K ,
- Juhl C , et al
- Higgins JPT ,
- Sweetman EA ,
- ↵ Tracking switched outcomes in clinical trials. compare . Available: http://compare-trials.org [Accessed 1 Dec 2021 ].
- Goldacre B ,
- Drysdale H ,
- Dale A , et al
- Marston C , et al
- Schulz KF ,
- Hoffmann TC ,
- Glasziou PP
- Glasziou PP ,
- Boutron I , et al
- Dionne CE ,
- Underwood M , et al
- Webster RK ,
- Rees JL , et al
- Julious SA ,
- Sones W , et al
- Krishan A ,
- Stocken D , et al
- Mansournia MA ,
- Collins GS ,
- Nielsen RO , et al
- Dumville JC ,
- Torgerson DJ ,
- Lysdal FG ,
- Tolstrup JS , et al
- Clausen MB ,
- Hölmich P ,
- Thorborg K ,
- Paavola M ,
- Malmivaara A ,
- Taimela S , et al
- Weissgerber TL ,
- Winham SJ , et al
- Pocock SJ ,
- Travison TG ,
- Hodkinson A ,
- Kirkham JJ ,
- Tudur-Smith C , et al
- Ioannidis JPA ,
- Evans SJW ,
- Gøtzsche PC , et al
- ↵ Data sharing in clinical trials: keeping score. The BMJ , 2019 . Available: https://blogs.bmj.com/bmj/2019/07/10/data-sharing-in-clinical-trials-keeping-score/ [Accessed 1 Dec 2021 ].
- Banks GC , et al
- ↵ Medical Research Council MRC. data sharing , 2020 . Available: https://mrc.ukri.org/research/policies-and-guidance-for-researchers/data-sharing/ [Accessed 1 Dec 2021 ].
- Hrynaszkiewicz I ,
- Norton ML ,
- Vickers AJ , et al
- Committee on Strategies for Responsible Sharing of Clinical Trial Data, Board on Health Sciences Policy, Institute of Medicine
- Milne G , et al
- ↵ Centre for Journalology . Available: http://www.ohri.ca/auditfeedback [Accessed 6 Dec 2021 ].
- Bourne PE ,
- Vale RD , et al
- ↵ List of academic journals by preprint policy. Wikipedia , 2020 . Available: https://en.wikipedia.org/w/index.php?title=List_of_academic_journals_by_preprint_policy&oldid=966674073 [Accessed 1 Dec 2021 ].
- ↵ Article sharing . Available: https://www.elsevier.com/about/policies/sharing [Accessed 1 Dec 2021 ].
- Silva D de O ,
- Taborda B ,
- Pazzinatto M
- Araujo AC ,
- Nascimento DP , et al
- ↵ The conversation: in-depth analysis, research, news and ideas from leading Academics and researchers. The conversation . Available: https://theconversation.com/uk [Accessed 1 Dec 2021 ].
- ↵ How to get your research in the media – nest . Available: https://www.latrobe.edu.au/nest/get-research-media/ [Accessed 1 Dec 2021 ].
Twitter @TBandholm, @KThorborg, @clare_ardern, @drrchristensen, @henriksen_mh
Contributors Conception and design: TB. Drafting and critical revision for important intellectual content: all authors. Final approval of manuscript. all authors. Accountable for all aspects of the work: all authors.
Funding CLA has received research salary support for work in return to sport from the Australian National Health and Medical Research Council (Early Career Fellowship APP1109779), Swedish Research Council (2015-03687) and Swedish Research Council for Sport Science (Postdoctoral fellowship D2018-0041), and is Editor-in-Chief of JOSPT (Journal of Orthopaedic & Sports Physical Therapy). The Parker Institute, Bispebjerg and Frederiksberg Hospital is supported by a core grant from the Oak Foundation (OCAY-18-774-OFIL).
Competing interests CLA was Deputy Editor (Systematic Reviews) for BJSM from 2016 to 2018 and is currently the Editor-in-Chief for JOSPT. TB and KT are BJSM editorial board members.
Provenance and peer review Not commissioned; externally peer reviewed.
Read the full text or download the PDF:
- Article Information
Data Sharing Statement
- As Ozempic’s Popularity Soars, Here’s What to Know About Semaglutide and Weight Loss JAMA Medical News & Perspectives May 16, 2023 This Medical News article discusses chronic weight management with semaglutide, sold under the brand names Ozempic and Wegovy. Melissa Suran, PhD, MSJ
- Patents and Regulatory Exclusivities on GLP-1 Receptor Agonists JAMA Special Communication August 15, 2023 This Special Communication used data from the US Food and Drug Administration to analyze how manufacturers of brand-name glucagon-like peptide 1 (GLP-1) receptor agonists have used patent and regulatory systems to extend periods of market exclusivity. Rasha Alhiary, PharmD; Aaron S. Kesselheim, MD, JD, MPH; Sarah Gabriele, LLM, MBE; Reed F. Beall, PhD; S. Sean Tu, JD, PhD; William B. Feldman, MD, DPhil, MPH
- What to Know About Wegovy’s Rare but Serious Adverse Effects JAMA Medical News & Perspectives December 12, 2023 This Medical News article discusses Wegovy, Ozempic, and other GLP-1 receptor agonists used for weight management and type 2 diabetes. Kate Ruder, MSJ
- GLP-1 Receptor Agonists and Gastrointestinal Adverse Events—Reply JAMA Comment & Response March 12, 2024 Ramin Rezaeianzadeh, BSc; Mohit Sodhi, MSc; Mahyar Etminan, PharmD, MSc
- GLP-1 Receptor Agonists and Gastrointestinal Adverse Events JAMA Comment & Response March 12, 2024 Karine Suissa, PhD; Sara J. Cromer, MD; Elisabetta Patorno, MD, DrPH
- GLP-1 Receptor Agonist Use and Risk of Postoperative Complications JAMA Research Letter May 21, 2024 This cohort study evaluates the risk of postoperative respiratory complications among patients with diabetes undergoing surgery who had vs those who had not a prescription fill for glucagon-like peptide 1 receptor agonists. Anjali A. Dixit, MD, MPH; Brian T. Bateman, MD, MS; Mary T. Hawn, MD, MPH; Michelle C. Odden, PhD; Eric C. Sun, MD, PhD
- Glucagon-Like Peptide-1 Receptor Agonist Use and Risk of Gallbladder and Biliary Diseases JAMA Internal Medicine Original Investigation May 1, 2022 This systematic review and meta-analysis of 76 randomized clinical trials examines the effects of glucagon-like peptide-1 receptor agonist use on the risk of gallbladder and biliary diseases. Liyun He, MM; Jialu Wang, MM; Fan Ping, MD; Na Yang, MM; Jingyue Huang, MM; Yuxiu Li, MD; Lingling Xu, MD; Wei Li, MD; Huabing Zhang, MD
- Cholecystitis Associated With the Use of Glucagon-Like Peptide-1 Receptor Agonists JAMA Internal Medicine Research Letter October 1, 2022 This case series identifies cases reported in the US Food and Drug Administration Adverse Event Reporting System of acute cholecystitis associated with use of glucagon-like peptide-1 receptor agonists that did not have gallbladder disease warnings in their labeling. Daniel Woronow, MD; Christine Chamberlain, PharmD; Ali Niak, MD; Mark Avigan, MDCM; Monika Houstoun, PharmD, MPH; Cindy Kortepeter, PharmD
See More About
Select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Others Also Liked
- Download PDF
- X Facebook More LinkedIn
Sodhi M , Rezaeianzadeh R , Kezouh A , Etminan M. Risk of Gastrointestinal Adverse Events Associated With Glucagon-Like Peptide-1 Receptor Agonists for Weight Loss. JAMA. 2023;330(18):1795–1797. doi:10.1001/jama.2023.19574
Manage citations:
© 2024
- Permissions
Risk of Gastrointestinal Adverse Events Associated With Glucagon-Like Peptide-1 Receptor Agonists for Weight Loss
- 1 Faculty of Medicine, University of British Columbia, Vancouver, British Columbia, Canada
- 2 StatExpert Ltd, Laval, Quebec, Canada
- 3 Department of Ophthalmology and Visual Sciences and Medicine, University of British Columbia, Vancouver, Canada
- Medical News & Perspectives As Ozempic’s Popularity Soars, Here’s What to Know About Semaglutide and Weight Loss Melissa Suran, PhD, MSJ JAMA
- Special Communication Patents and Regulatory Exclusivities on GLP-1 Receptor Agonists Rasha Alhiary, PharmD; Aaron S. Kesselheim, MD, JD, MPH; Sarah Gabriele, LLM, MBE; Reed F. Beall, PhD; S. Sean Tu, JD, PhD; William B. Feldman, MD, DPhil, MPH JAMA
- Medical News & Perspectives What to Know About Wegovy’s Rare but Serious Adverse Effects Kate Ruder, MSJ JAMA
- Comment & Response GLP-1 Receptor Agonists and Gastrointestinal Adverse Events—Reply Ramin Rezaeianzadeh, BSc; Mohit Sodhi, MSc; Mahyar Etminan, PharmD, MSc JAMA
- Comment & Response GLP-1 Receptor Agonists and Gastrointestinal Adverse Events Karine Suissa, PhD; Sara J. Cromer, MD; Elisabetta Patorno, MD, DrPH JAMA
- Research Letter GLP-1 Receptor Agonist Use and Risk of Postoperative Complications Anjali A. Dixit, MD, MPH; Brian T. Bateman, MD, MS; Mary T. Hawn, MD, MPH; Michelle C. Odden, PhD; Eric C. Sun, MD, PhD JAMA
- Original Investigation Glucagon-Like Peptide-1 Receptor Agonist Use and Risk of Gallbladder and Biliary Diseases Liyun He, MM; Jialu Wang, MM; Fan Ping, MD; Na Yang, MM; Jingyue Huang, MM; Yuxiu Li, MD; Lingling Xu, MD; Wei Li, MD; Huabing Zhang, MD JAMA Internal Medicine
- Research Letter Cholecystitis Associated With the Use of Glucagon-Like Peptide-1 Receptor Agonists Daniel Woronow, MD; Christine Chamberlain, PharmD; Ali Niak, MD; Mark Avigan, MDCM; Monika Houstoun, PharmD, MPH; Cindy Kortepeter, PharmD JAMA Internal Medicine
Glucagon-like peptide 1 (GLP-1) agonists are medications approved for treatment of diabetes that recently have also been used off label for weight loss. 1 Studies have found increased risks of gastrointestinal adverse events (biliary disease, 2 pancreatitis, 3 bowel obstruction, 4 and gastroparesis 5 ) in patients with diabetes. 2 - 5 Because such patients have higher baseline risk for gastrointestinal adverse events, risk in patients taking these drugs for other indications may differ. Randomized trials examining efficacy of GLP-1 agonists for weight loss were not designed to capture these events 2 due to small sample sizes and short follow-up. We examined gastrointestinal adverse events associated with GLP-1 agonists used for weight loss in a clinical setting.
We used a random sample of 16 million patients (2006-2020) from the PharMetrics Plus for Academics database (IQVIA), a large health claims database that captures 93% of all outpatient prescriptions and physician diagnoses in the US through the International Classification of Diseases, Ninth Revision (ICD-9) or ICD-10. In our cohort study, we included new users of semaglutide or liraglutide, 2 main GLP-1 agonists, and the active comparator bupropion-naltrexone, a weight loss agent unrelated to GLP-1 agonists. Because semaglutide was marketed for weight loss after the study period (2021), we ensured all GLP-1 agonist and bupropion-naltrexone users had an obesity code in the 90 days prior or up to 30 days after cohort entry, excluding those with a diabetes or antidiabetic drug code.
Patients were observed from first prescription of a study drug to first mutually exclusive incidence (defined as first ICD-9 or ICD-10 code) of biliary disease (including cholecystitis, cholelithiasis, and choledocholithiasis), pancreatitis (including gallstone pancreatitis), bowel obstruction, or gastroparesis (defined as use of a code or a promotility agent). They were followed up to the end of the study period (June 2020) or censored during a switch. Hazard ratios (HRs) from a Cox model were adjusted for age, sex, alcohol use, smoking, hyperlipidemia, abdominal surgery in the previous 30 days, and geographic location, which were identified as common cause variables or risk factors. 6 Two sensitivity analyses were undertaken, one excluding hyperlipidemia (because more semaglutide users had hyperlipidemia) and another including patients without diabetes regardless of having an obesity code. Due to absence of data on body mass index (BMI), the E-value was used to examine how strong unmeasured confounding would need to be to negate observed results, with E-value HRs of at least 2 indicating BMI is unlikely to change study results. Statistical significance was defined as 2-sided 95% CI that did not cross 1. Analyses were performed using SAS version 9.4. Ethics approval was obtained by the University of British Columbia’s clinical research ethics board with a waiver of informed consent.
Our cohort included 4144 liraglutide, 613 semaglutide, and 654 bupropion-naltrexone users. Incidence rates for the 4 outcomes were elevated among GLP-1 agonists compared with bupropion-naltrexone users ( Table 1 ). For example, incidence of biliary disease (per 1000 person-years) was 11.7 for semaglutide, 18.6 for liraglutide, and 12.6 for bupropion-naltrexone and 4.6, 7.9, and 1.0, respectively, for pancreatitis.
Use of GLP-1 agonists compared with bupropion-naltrexone was associated with increased risk of pancreatitis (adjusted HR, 9.09 [95% CI, 1.25-66.00]), bowel obstruction (HR, 4.22 [95% CI, 1.02-17.40]), and gastroparesis (HR, 3.67 [95% CI, 1.15-11.90) but not biliary disease (HR, 1.50 [95% CI, 0.89-2.53]). Exclusion of hyperlipidemia from the analysis did not change the results ( Table 2 ). Inclusion of GLP-1 agonists regardless of history of obesity reduced HRs and narrowed CIs but did not change the significance of the results ( Table 2 ). E-value HRs did not suggest potential confounding by BMI.
This study found that use of GLP-1 agonists for weight loss compared with use of bupropion-naltrexone was associated with increased risk of pancreatitis, gastroparesis, and bowel obstruction but not biliary disease.
Given the wide use of these drugs, these adverse events, although rare, must be considered by patients who are contemplating using the drugs for weight loss because the risk-benefit calculus for this group might differ from that of those who use them for diabetes. Limitations include that although all GLP-1 agonist users had a record for obesity without diabetes, whether GLP-1 agonists were all used for weight loss is uncertain.
Accepted for Publication: September 11, 2023.
Published Online: October 5, 2023. doi:10.1001/jama.2023.19574
Correction: This article was corrected on December 21, 2023, to update the full name of the database used.
Corresponding Author: Mahyar Etminan, PharmD, MSc, Faculty of Medicine, Departments of Ophthalmology and Visual Sciences and Medicine, The Eye Care Center, University of British Columbia, 2550 Willow St, Room 323, Vancouver, BC V5Z 3N9, Canada ( [email protected] ).
Author Contributions: Dr Etminan had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Sodhi, Rezaeianzadeh, Etminan.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Sodhi, Rezaeianzadeh, Etminan.
Critical review of the manuscript for important intellectual content: All authors.
Statistical analysis: Kezouh.
Obtained funding: Etminan.
Administrative, technical, or material support: Sodhi.
Supervision: Etminan.
Conflict of Interest Disclosures: None reported.
Funding/Support: This study was funded by internal research funds from the Department of Ophthalmology and Visual Sciences, University of British Columbia.
Role of the Funder/Sponsor: The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Data Sharing Statement: See Supplement .
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
- Using the CRA Homepage in CTMS
- Using the EDC Connection in CTMS
- Using the Study Manager Homepage in CTMS
- Working with Trip Reports in CTMS
- Japanese Clinical Trial Notifications in CTMS
- eTMF Overview
- About the Model & Artifact Objects (eTMF & CTMS)
- Archiving Studies in eTMF
- Auto-Filing Documents in TMF Binders
- Bulk Creating Study Binders (eTMF)
- Configuring for Study Archival in eTMF
- Configuring the TMF Homepage
- Configuring the TMF Viewer
- Configuring Quality Issues (eTMF & Study Startup)
- Managing Locations
- Managing Studies with Lifecycles in eTMF
- Managing the TMF Index
- Setting Up CRF Import in eTMF
- Setting up TMF Transfer
- TMF Homepage
- TMF Transfer
- Using CRF Import in eTMF
- Working with EDLs
- Configuring EDLs (Clinical Operations)
- Evaluating TMF Bot Auto-classification Models
- TMF Bot FAQ (eTMF)
- Training Auto-classification Models for TMF Bot (eTMF)
- Using the TMF Bot (eTMF)
- Evaluating Metadata Extraction Models
- Testing Metadata Extraction Models
- Using TMF Bot Document Quality Control
- Vault Payments Overview
- Configuring for Study Budget Tracking
- Configuring Vault Payments
- Setting Up Studies for Site Payments
- Study Budget Tracking with Vault Payments
- Study Startup Overview
- About the Model & Artifact Objects (Study Startup)
- Auto-Filing Documents in Study Startup Binders
- Clinical Application Management
- Configuring Surveys for External Respondents
- Setting Up Site Activation Progress View
- Site Feasibility in Study Startup
- Using Site Activation Progress View (Study Startup)
- Using the Study Startup Specialist Homepage
- Working with Surveys for External Respondents
- Veeva Site Connect Overview
- Configuring Document Reconciliation (Veeva Site Connect)
- Configuring Safety Distributions
- Configuring Veeva Site Connect
- Distributing Safety Documents with Veeva Site Connect
- Document Reconciliation (Veeva Site Connect)
- Document Transfer Behavior with Veeva Site Connect
- Sending Documents & Document Requests (Veeva Site Connect)
- Setting Up Study Site Agreements for Veeva Site Connect
- SiteVault Inviter
- Veeva eConsent Overview
- Configuring Veeva eConsent Authoring (Clinical Operations)
- Veeva eConsent Authoring (Clinical Operations)
- Using the Veeva eConsent Editor
- Veeva ePRO Overview
- Using MyVeeva Studio
- Using a Collection
- Using Survey Libraries
- Configuring Surveys
- Configuring Conditions
- Configuring Scores
- Managing Groups
- Managing Events
- Configuring Schedules and Notifications
- Managing Languages and Translations
- Performing User Acceptance Testing (UAT) on a Collection
- Approving, Upversioning, and Deleting a Collection
- Connecting a SiteVault to Your Collection
- Exporting Survey, Adherence, and Audit Trail Data
- Changing Survey Data
- Sample JSON Survey, Schedule, and Notification Templates
Trip Report Administration (CTMS)
With Vault, you can manage your users’ monitoring trips and information with CTMS Trip Reports. This feature collects all trip information into a single Vault object, Monitoring Event , and enables users to automatically format it into a Monitoring Visit Report (MVR) document. Vault uses formatted output to create documents for your Monitoring Visit Reports .
You can manage these objects and documents using Trip Report Templates . This template object brings together trip report details, as well as question and answer sets for a visit. When you use a Trip Report Template to generate questions on a Monitoring Event , Vault adds the Trip Report Template name to the Monitoring Event record giving you additional tracking and reporting abilities.
Note : This feature is only available on Clinical Operations CTMS Vaults.
Enabling Trip Reports
Contact Veeva Support to enable trip reports in your Vault.
Recommended Configuration
To effectively use trip reports, we recommend you perform the following configuration tasks in your Vault.
Lifecycle & Workflow Configuration
The CTMS Trip Reports feature provisions the necessary changes to your Trip Report Template and Monitoring Event lifecycles and workflows. However, you will need to set the new lifecycle’s Status to Active and make configuration changes as needed to best suit the needs of your organization.
- To use the Plan Monitoring Event workflow, configure your Monitoring Event lifecycle’s Expected state to include a Workflow type user action to start the Plan Monitoring Event workflow.
- To use the Create Monitoring Confirmation Letter workflow, configure your Monitoring Event lifecycle’s In Progress and Confirmed states to include a Workflow type user action to start the Create Monitoring Confirmation Letter workflow.
- To use the Create Monitoring Follow Up Letter workflow, configure your Monitoring Event lifecycle’s In Progress, Ready for Review , In Review , Passed Review , In Signature , and Final states to include a Workflow type user action to start the Create Monitoring Event Follow Up Letter workflow.
- To seed Monitored Subjects and Monitored Subject Visits , configure your Monitoring Event lifecycle’s In Progress state to include the Seed Monitored Enrollment or Proactively Seed Monitored Enrollment entry action to seed Monitored Enrollment data when a Monitoring Event begins. Add the Seed Monitored Enrollment or Proactively Seed Monitored Enrollment user action to re-seed Monitored Enrollment data for visits that occur over multiple days.
- Configure the Monitoring Event lifecycle to seed Monitoring Follow Up Items into Monitoring Events records .
- Configure the Monitoring Event lifecycle to seed Issues into Monitoring Events records .
- Configure the Review & Approve Monitoring Event workflow to ensure all required responses, comments, follow up items, and issues are populated .
- Configure the Review Comments are Resolved entry action on the Monitoring Event lifecycle to ensure that all Review Comments are resolved before the workflow progresses, including orphaned Review Comments that are hidden by Vault. Review Comments can become orphaned when a user creates one for a record, then deletes the record while the Review Comment is still open.
Trip Report Formatted Output
This feature includes a formatted output template that Vault uses to create PDFs of trip reports. If any additional information is required to include in a trip report document, you can update your formatted output template .
Configuring Long Text Fields
The default setting for question responses and comments limits text fields to 1,500 characters. To allow users to enter up to 32,000 characters, navigate to Admin > Settings > Application Settings and set the Enable Long Text Trip Report Questions checkbox.
By default, the maximum length of the long text fields is 5,000. You can adjust this setting by changing the Maximum Length value of the Response Text and Comment long text fields on the Trip Report Question Response object.
Migrating to Long Text Fields
If you already have existing Monitoring Events in your Vault, enabling long text fields requires a small data migration. Contact your Veeva services representative for help with the following steps:
- Enable Configuration Mode to ensure users are not creating or updating Trip Report Question Responses while you perform this migration.
- Navigate to Vault Loader and Extract the Trip Report Question Responses object records.
- Copy the data in the response_text__ctms column to the long_response_text__v column.
- Copy the data in the comment__ctms column to the long_comment__v column.
- Delete all columns except id , long_response_text__v and long_comment__v .
- Save the CSV file.
- In Vault Loader, Choose the CSV File.
- Select the Trip Report Question Responses Object Type .
- Select the Update Action Type .
- Select the ID Key Field .
- Click Start Load .
Creating Trip Report Templates & Uploading Questions
Configuration for uploading trip report questions.
You can configure the following user actions on the Draft state of the Trip Report Template object lifecycle, which allow you to quickly populate trip report questions in bulk:
- Workflow > Trip Report Template Review
- Download Trip Report Template
- Upload Trip Report Questions
Note that these user actions may already be configured in new CTMS Vaults.
Creating Answer Sets
Before you can create your Trip Report Template and upload Questions , you must create Answer Set records. You can create these records manually from the Trip Report Template Admin > Answer Set Bank tab, or load them in bulk using Vault Loader . When you create Answer Sets , you must create individual Answer records in the Available Answers section of the Answer Set record detail page. For example, when you create a “Yes / No” Answer Set, you must create two Answer records: Yes and No.
Answer sets exist separately from your Trip Report Templates . You can create a large bank of answers in your Vault to use with multiple templates. When you upload or create multiple choice Questions , Vault associates each question to an Answer Set using an object reference field.
When creating Answer Sets , make sure to include an Order to ensure that the answers are displayed in a specific sequence.
Creating Trip Report Templates
You can create Trip Report Templates for each type of monitoring visit your CRAs might perform: Interim, Oversight, Pre Study, Site Close Out, and Site Initiation. When a CRA starts authoring a trip report, Vault will preferentially select an approved template with the nearest match on visit type, study and study country.
You can create a new Trip Report Template to upload a new set of questions, or copy an existing Trip Report Template to copy over a base set of questions for the visit type and customize the new template for your study and study country.
To create a new Trip Report Template , navigate to Trip Report Template Admin > Trip Report Templates and click Create . Select a Trip Report template type in the dialog, then click Continue . Enter the record details and click Save . Note that selecting a Study or a Study Country creates a study-specific trip report. Do not enter values for these fields unless you intend to use the template for a specific study.
To copy and customize an existing Trip Report Template , choose a template of the desired visit type and select Copy Record from the Actions menu. Fill in Study and Study Country as needed and Save . When prompted, check the box to Copy Related Records . This will copy your base set of questions for the visit type. You can then add questions manually , delete existing questions, or change the question Order to customize your new template.
Uploading Questions to New Trip Report Templates
To add Questions to a new Trip Report Template , select Download Trip Report Template from the Actions menu. Vault automatically downloads a CSV template file you can use to populate questions, instructions, conditional requirements, and dependent questions.
Note : If you run the Download Trip Report Template action and the template is blank, Vauls downloads a blank CSV template file. If data is present on the template, Vault downloads the CSV template file populated with data.
This table describes the columns in the CSV file:
All CSV values are case-sensitive and must exactly match values in your Vault, except for the Yes/No (boolean) columns.
When you finish populating your CSV, click Upload Trip Report Questions from the Trip Report Template record’s Actions menu and select your file. Vault displays an error message detailing what went wrong. Note that you must use UTF-8 encoding in your CSV file in order for it to upload successfully.
Vault automatically creates a Question record and a Trip Report Question Template record for each row in your CSV file and groups your Questions into the Trip Report Sections you defined. If the Trip Report Section you entered doesn’t exist, Vault automatically creates a new Trip Report Section record.
Once you upload your Questions , you can edit the Order for a question from the Template Questions section. The number you enter in the Order field displays with your Question on the Monitoring Event Details page and in the trip report.
Approving Your Templates
When you have finished creating or modifying your template, select the option to approve the template from the Actions menu.
Creating Questions Manually
You can also create individual Questions separately from a Trip Report Template , and associate individual Questions to your Trip Report Template during template creation later. You can create these Question records from the Trip Report Template Admin > Question Bank tab, or load them in bulk using Vault Loader.
Before you create your Questions , navigate to the Trip Report Section object in Admin > Business Admin and create any new Trip Report Section records in which to group your Questions . Enter a Name and select a Section Type from the picklist. Vault does not allow you to create records with duplicate values. We recommend including the trip report type in the record Name , for example, “IMV – Trial Study Requirements” and “COV – Trial Study Requirements.” You can configure the Trip Report Section Type picklist from the Business Admin > Picklists page.
After you’ve created your Trip Report Sections , create your Question records and fill in the relevant fields. To ensure that a Response is required, select Yes for Required Response . To group a Question into a Trip Report Section , choose the desired section from the Trip Report Section field on the Question record. If configured , you can enter Instructions that will be displayed to users to explain how they should answer the Question .
When you’ve created all Questions , you can add them to your Trip Report Template . Navigate to your Trip Report Template record and click Create in the Template Questions section. Select a Section , the Question , and specify an Order for the question.
Creating Questions with Instructions
You can enter Instructions that Vault displays to users to explain how they should answer the Question . To do this, you must first add the Instructions field to the Details section of the Question page layout.
Creating Questions with Conditional Requirements
For multiple-choice Question Types , you can require users to enter comments and log follow up items, including issues to follow up on, based on which answer they select. For example, if you create a question to ask, “Was the protocol amended since the last visit?” with possible answers of Yes and No , you can specify that users must enter a comment if they select Yes but not if they select No , and that users must log a follow up item if they select No but not if they select Yes .
Creating Dependent Questions
Dependent questions can only be answered if a specific answer is chosen for a multiple-choice controlling question. This is similar to having conditional requirements, but instead of adding additional items to the question, a new question becomes editable. This allows for branching lines of questioning in your Trip Reports.
To make a question dependent on a previous answer, select the Controlling Question . The control field for Controlling Answer shows the available answers for that question. Choose the Controlling Answer . The question you are editing will only become editable in the Trip Report if the Controlling Answer is chosen for the Controlling Question .
When a user answers the Controlling Question with the Controlling Answer , Vault automatically displays the dependent question as editable. Similarly, if a user changes the Controlling Question response to a response that is not the Controlling Answer , Vault automatically displays any dependent questions as uneditable. This works within the same Trip Report Section or across sections.
Object Configurations
Page layout configurations.
You must first configure the following object page layouts before you can create dependent questions, or make comments, follow up items, and issues required:
- On the Question object page layout, add the Answer with Conditional Requirements related object section so that you can specify which answers require comments, follow up items, and issues.
- Required Comment
- Follow Up Item Requiredness
- Issue Requiredness
- Controlling Question
- Check the box for Specify different Label and Help Content
- Update the label to Controlling Answer
- Controlling Answer
- Dependent Question Behavior
On the Answer with Conditional Requirements object, unselect the Relate Multiple Records setting to change the object from a simple to a complex join .
How to Make Comments, Follow Up Items & Issues Required
- Navigate to a multiple-choice Question record.
- Click Create in the Answer with Conditional Requirements section.
- Select the predefined answers that will require comments, follow up items, or issues.
- Enter values for the appropriate fields and click SAVE .
- Optional: Configure the Verify Required Trip Report Questions system action to ensure all required responses, comments, follow up items, and issues are populated before users can proceed to the next lifecycle state. If the workflow step criteria are not met, Vault displays a dialog to the user listing out the sections that are missing required values. Vault populates the question response as Not Evaluated - Dependent Question when locked dependent questions are not answered.
As of the 21R2.2 release, Vault uses the Highlight Required field on Trip Report Question Response records to highlight required fields. Prior to this release, Vault checked the Highlight Required field on related Monitoring Event records; this field is now deprecated. No configuration changes are necessary to accommodate this change.
Note : As of the 21R1.3 release, the Required Comment field on Answer with Conditional Requirement ( question_comment_required__v ) object must be set to Yes in order for comments to be required for the related answer. As part of this change, the Required Comment field was automatically set to Yes on all Answer with Conditional Requirements records in your Vault.
Prior to the 21R1.3 release, answers with required comments were determined by related Answer with Conditional Requirements records.
Adding Questions to Monitoring Events
Vault groups your Questions into the Trip Report Sections you define, which you can then add to your Monitoring Event page layout . Vault automatically populates that page layout section with the related Questions .
To add a Trip Report Section to the page layout of your Monitoring Event record:
- Navigate to Configuration > Objects > Monitoring Event > Page Layouts .
- Click to open the page layout.
- For Add Section , select Trip Report Questions .
- Enter a Label for the section.
- Optional: To display this section only when the Monitoring Event record is in certain lifecycle states, select states from the Show the section only in these lifecycle states drop-down.
- Select a Section Type . This list shows the Trip Report Section records in your Vault.
- Click Done .
- Optional: Click and drag your new section to reorder.
- Click Save .
Configuring Reviewer Comments
Reviewer comments enable you to easily provide feedback on monitoring events. See Working with Trip Reports (CTMS) for general information about reviewer comments.
Configuration Overview
- Add the Review Status column to the following page layout sections of a Monitoring Event : Monitoring Event Participants , Monitored Metrics (Enrollment Metrics) , Monitored Subject (Subject Data) , Monitored Subject Visit (Subject Visit SDV) , Monitored ICFs , Protocol Deviations , and Clinical User Tasks . Add the Review Status column to these sections to allow users to provide feedback on monitoring event details via reviewer comments.
- Select the Enable Monitoring Reviewer Comments checkbox from Admin > Settings > Application Settings. Vault will automatically add the Review Status column to all question sections on the Monitoring Event object. Note that this checkbox does not affect the Review Status column on any other section.
- Ensure relevant permission sets have Read , Edit , Create , and Delete permissions on the Review Comment object and Read permission on the Review Summary object.
Defaulting Activities & Participants
When configured , Vault automatically adds Monitoring Event Participants and Monitoring Activities , via a system action, when a CRA plans a Monitoring Event .
Vault adds site personnel with the Site Monitor or Principal Investigator roles as invited Monitoring Event Participants by default. Vault also automatically adds Monitoring Activities to the visit, based on the standard Monitoring Activities for the selected visit type (object types for the Monitoring Event object).
When users select Plan Visit from the Monitoring Event record’s Actions menu, the system action creates and relates the appropriate Monitoring Event Participants and Monitoring Activities . This action also changes the Monitoring Event record’s lifecycle state from Expected to Planning .
Seeding Monitored Subjects & Monitored Subject Visits
You can configure Vault to seed Monitored Subjects and Monitored Subject Visits using the Seed Monitoring Enrollment or Proactively Seed Monitoring Enrollment user or entry actions. Seeding data reduces data entry for CRAs.
Monitored Subjects
When configured , Vault automatically seeds the Monitored Subjects object with a copy of the current Subjects and their statuses for the Site .
Monitored Subject Visits
When configured , Vault automatically seeds the Monitored Subjects object with a copy of the current Subject Visits that apply to the Subjects created in the Monitored Subject . See details about when Vault seeds Monitored Subject Visit data .
Seeding Follow-up Items
You can configure Vault to seed follow-up items related to your monitoring event to use in trip reports or report letters.
To enable this:
- Navigate to Admin > Settings > Application Settings and click Edit .
- In the CTMS Features section, set the Remove Follow Up Sections option and click Save . This removes the Open Follow-Up Items and Closed Since Last Follow-Up Items sections and adds new sections for the Monitored Open Follow-Up Items and Monitored Closed Since Last Follow-Up Items join objects. You cannot undo this action.
- Configure the Seed Follow-Up Items system action in a workflow on the Monitoring Event lifecycle.
- Add related object sections to the Monitoring Event page layout for the Monitored Open Follow-Up Item > Clinical User Task and Monitored Closed Follow-Up Item > Clinical User Task objects.
- Add the Seed Follow-Up Items user action to the applicable states of the Monitoring Event lifecycle.
Seeding Issues
You can configure Vault to seed issues related to your monitoring event to use in trip reports or report letters.
- Configure the Seed Issues system action in a workflow on the Monitoring Event lifecycle.
- Add two related object sections, Open Issues and Closed Issues Since Last Visit , to the Monitoring Event page layout for the Monitored Open Issue > Issue object. On the Open Issues section, set Criteria VQL on the page layout to filter issues where Resolved Date is blank. On the Closed Issues Since Last Visit section, set Criteria VQL on the page layout to filter issues where Resolved Date is not blank.
- Add the Seed Issues entry action and user action to the applicable states of the Monitoring Event lifecycle.
Custom Monitoring Event Types
You can configure custom Monitoring Event types using object types . If you choose to use custom Monitoring Event types, perform the following configuration tasks to match the functionality of the standard Monitoring Event types:
- Monitoring Visit Confirmation Letter
- Monitoring Visit Follow Up Letter
- Milestone Type
- Trip Report
- Create document templates for confirmation and follow up letters for this Monitoring Event type.
- Add a system action step to the Create Monitoring Confirmation Letter workflow for the new Monitoring Event type. Place the step after the Route Template by Visit Type decision step.
- Add a system action step to the Create Monitoring Follow Up Letter workflow for the new Monitoring Event type. Place the step after the Route Template by Visit Type decision step.
If the Milestone Type you select matches the object type of your custom monitoring event, Vault automatically creates a Milestone record of the correct type.
For example, if you added a Monitoring Event object type for a Remote Monitoring Event named “remote_monitoring_event__c” and you created a picklist value in the Milestone Type picklist labeled “Remote Monitoring Event” with the name “remote_monitoring_event__c”, and then you created a Monitoring Event of the object type Remote Monitoring Event , Vault would create a milestone with the milestone type of Remote Monitoring Event , and associate it to your Monitoring Event .
Related Permissions
You can complete all steps in this article with the standard System Admin or Vault Owner profile.
If your Vault uses custom security profiles, your profile must grant the following permissions:

GxP Lifeline
How to write a useful clinical monitoring report.

Documentation is among the least glamorous and most burdensome aspects of compliance . For regulators, nothing happened if it wasn’t documented. Some companies err on the side of caution by requiring too much documentation without focusing on the documentation’s purpose. However, it’s also important to not neglect documentation when it is needed. One place where this balance is particularly important is in site clinical monitoring .
Site monitoring reports serve a regulatory purpose by proving the site monitoring happened. Their usefulness extends beyond simply checking a box on the compliance checklist. A well-written report shouldn’t be an afterthought but should be an integral part of the site visit. The report and visit are essential parts of proper clinical trial management .
#1: Clinical Monitoring Report Preparation
Site monitoring has a purpose. Keep that purpose in mind as you prepare. There are requirements in the study protocol that must be met, some of which pertain to what information needs to be included in the report. Depending on how or if the clinical trial is using centralized or remote monitoring, some of the information for the report can be collected before the monitor is even on-site. If those methods are used, reviewing documentation and data can be done beforehand. Remote access to that information is also useful when it comes to writing the report after the visit.
An important component of preparing is to look at past clinical monitoring reports and audit reports, if any have been conducted. If a previous site interim visit reported a problem, that requires follow-up during the next visit. Time on-site is limited, so these issues should be prioritized. A risk-based approach to monitoring will also indicate certain areas that require precedent.
#2: Note Taking
Taking notes takes time, but it’s worth it in situations where you couldn’t possibly remember everything. The monitoring report template should be open throughout the visit, both to keep you on track and to give you a place to write notes. Everyone has their own system of taking notes, but your focus should be on the areas most important to regulators — patient safety and data quality. This is the whole reason for doing the site monitoring, so take notes that demonstrate adherence to protocol, good clinical practices (GCP) , and regulations. Or notes on how the site is not adhering to those requirements.
#3: Monitoring Follow-Up
Your clinical monitoring visit and the ensuing report aren’t isolated incidents. They will be shaped by previous visits and affect future ones. Hopefully, issues from the past site monitoring reports that you reviewed while preparing for the visit have been resolved. If not, include it in your notes and the final report. Any corrective action the site has implemented should prevent recurrence. The site and monitor should work together to find solutions that will bring the site into compliance. Your report should also include any items that will need follow-up during the next visit.
Besides follow-up for a single site, monitors should also be aware of how any issues might reflect a trend across the whole study. If multiple sites are having the same issue, it might have more to do with the study protocol than the sites themselves.
#4: Write Your Clinical Monitoring Report ASAP
Taking great notes is critical, but the best time to write the report is when it’s fresh on your mind. There might be details that didn’t make it into your notes that would soon be forgotten. Or you might look back at your notes later and realize something that made sense in the moment no longer does. It’s important to write the report quickly because human memory is fallible. Going along with that, if multiple site visits are scheduled for the same day, there’s danger in remembering something from one site as pertaining to another. At the very least, the report for one site should be written before visiting the next.
#5: Be Clear and Concise in Your Report
A site monitoring report shouldn’t read like a doctoral thesis. But it also can’t be a collection of bullet points. There’s a balance that monitors have to achieve between having too much information and not enough. The report absolutely should include:
- The date of the visit.
- Name of people involved (from the site and sponsor).
- Summary of what was reviewed.
- Description of any issues or potential issues (e.g., nonconformance, problems with data, etc.)
- Description of any corrective action, who is responsible for that action, and when that action will be completed.
While there’s no wordcount requirement for a site monitoring report, the summary and descriptions do need to be detailed enough to verify the monitoring plan was followed.
Documenting what happens during a clinical monitoring visit is just as important as the visit itself. It’s essential for showing the site is compliant with regulations and the study protocol. It also demonstrates the improvements sites make when issues are discovered and who is responsible for them. Reports from previous visits serve as a starting point for the next visit and ensure follow-up items aren’t forgotten. Keeping all this in mind, the site monitoring report needs to be given forethought and attention to make it a useful document.

Sarah Beale is a content marketing specialist at MasterControl in Salt Lake City, where she writes white papers, web pages, and is a frequent contributor to the company’s blog, GxP Lifeline. Beale has been writing about the life sciences and health care for over five years. Prior to joining MasterControl she worked for a nutraceutical company in Salt Lake City and before that she worked for a third-party health care administrator in Chicago. She has a bachelor’s degree in English from Brigham Young University and a master’s degree in business administration from DeVry University.
An official website of the United States government
Here’s how you know
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.

U.S. Dept. of Health & Human Services
Clinical Research Study Investigator's Toolbox
The purpose of the NIA Clinical Research Toolbox is to provide a Web-based informational repository for investigators and staff involved in clinical research. The Toolbox contains templates, sample forms, guidelines, regulations and informational materials to assist investigators in the development and conduct of high quality clinical research studies.
Issued by: National Institutes of Health (NIH)
NIA Clinical Research Investigator's Toolbox
Supporting clinical research, study startup.
- NIA Guidance on Clinical Trials
Forms and Templates
- Glossary of Terms
Data Safety and Monitoring
As depicted in the NIA Guidance on Clinical Trials , NIA is responsible for overseeing the data and safety monitoring of the clinical research it supports. Data and safety monitoring of a clinical trial is commensurate with the risks posed to the study participants and with the size and complexity of the study.
Applicants requesting support for any intervention study must complete "PHS Human Subjects and Clinical Trials Information" form of the SF424 (R&R), describe a data and safety monitoring plan (DSMP), which discusses the need for an independent data and safety monitoring body or justifies why such a body is not needed to monitor the study and proposes an alternative safety monitoring mechanism. For example, for a single-site, low risk study, the PI may propose a local safety monitor, while a multi-site, higher risk study might propose a Data and Safety Monitoring Board (DSMB).
- Data and Safety Monitoring Plan (DSMP) Template and Guidelines (MS Word, 37K) and DSMP Checklist (MS Word, 43K) were developed to assist investigators in preparation of a sound data and safety monitoring plan. All clinical trials require study-specific monitoring procedures to ensure participant safety and data integrity. The DSMP outlines procedures that investigators and study staff will follow when implementing a clinical trial. Investigators submitting grant applications for clinical trials are required to include a general description of the DSMP as part of the research grant application.
- Guideline for Budgeting for Data and Safety Monitoring Activities (MS Word, 25K) aids investigators in budgeting for an independent DSMB or a Safety Officer when preparing the budget section of a grant application.
Data Sharing
The National Institutes of Health (NIH) advocates making available to the public the results and accomplishments of the activities that it funds. NIH assures that research resources developed with public funds become readily available to the broader research community in a timely manner for further research, development, application, and secondary data analysis. The expectation is that this will lead to products and knowledge of benefit to public health. To ensure that future research can build on previous efforts and discoveries, the National Institutes of Health (NIH) has developed a data sharing policy effective October 1, 2003, for applicants seeking NIH funding of $500,000 or more in direct costs in any one year. The policy expects final research data, especially unique data, from NIH-supported research efforts be made available to the investigators. The NIH policy on data sharing applies to:
- Basic research, clinical studies, surveys, and other types of research supported by the NIH.
- Human subjects and laboratory research.
- Data not produced with NIH funding but used in an NIH-supported activity in some instances.
Investigators are expected to include in their grant application a brief description of how final research data will be shared, or explain why data-sharing is not possible (for example: human subject protection concerns). Please see NIH’s Example Plan (MS Word, 55K) for a template you may modify to fit the data you plan to share.
Initial Proposal Concept Form (MS Word, 39K) - This form should be used to advocate for an initiative by the Division of Geriatrics and Clinical Gerontology (DGCG) for a clinical trial or trials that exceed $2 million in direct costs in any year of funding. DGCG Clinical Trials Advisory Panel, a task force of the National Advisory Council on Aging (NACA), will evaluate the concept proposals in October – November of each Fiscal Year and will provide its recommendations to DGCG, NACA, and to the NIA Director on initiatives for large clinical trials.
Back to top
The clinical protocol is a document that describes how a clinical study will be conducted by detailing the objective(s), design, methodology, statistical considerations and organization of a clinical study, and describes methods used to ensure the safety of the study participants and integrity of the data collected.
Protocol (MS Word, 93K) - The Clinical Intervention Study Protocol Template outlines a clinical study protocol and provides guidance on important content to include in each section. The template can be downloaded as an MS Word file for adaptation by the study investigator.
Manual of Procedures
A Manual of Procedures (MOP) is a handbook that details a study’s conduct and operations as well as facilitates consistency in protocol implementation and data collection across study participants and sites. It operationalizes the study protocol and describes each step of the study and how it is to be executed. A copy of the MOP should be provided to each member of the Study Team. Ideally, the MOP would contain an adequate amount of detail that any individual(s) at any site(s) could run the study consistently with only the information contained in the MOP and its appendices.
Get resources to support your study recruitment
Visit NIA’s ADORE (Alzheimer’s and Dementia Outreach, Recruitment, and Engagement) Resources for a searchable collection of materials for clinical trials recruitment and retention.
The NIA recognizes the importance of a MOP and has developed documents to assist principal investigators in writing their study MOP. Investigators with a multi-site study are required to submit a MOP, while single-site study investigators are strongly encouraged to review the MOP and determine which sections are necessary in order to ensure the study procedures are performed as intended. The Guidelines below provide details on each section of the MOP, while the MOP Outlines are an overview listing the sections that are most relevant in those types of studies.
- Manual of Procedures (MOP) Outline – Multi-Site (MS Word, 30K)
- Manual of Procedures (MOP) Guidelines – Multi-Site (MS Word, 179K)
- Manual of Procedures (MOP) Outline – Single-Site (MS Word, 27K)
- Manual of Procedures (MOP) Guidelines - Single-Site (MS Word, 170K)
The following documents can also be found within the MOP template:
- Screening Log provides documentation of all individuals who were evaluated for participation in a research study. The log typically contains a unique identification number for each person screened along with individuals’ initials, age, gender, race and ethnicity, screening date, and eligibility status.
- Schedule of Events presents the activities that take place at each contact with the participant.
- Protocol Deviation Log provides participant-specific documentation of missed visits and other actions that deviate from the protocol.
Informed Consent
The consent process provides individuals with sufficient information for making informed decisions about participation in a clinical research study. The following documents are provided as a tool to assist NIA investigators for developing a comprehensive informed consent:
- Informed Consent Template (MS Word, 115K) provides a general outline of a study specific informed consent form (ICF). It is critical that investigators consult with their local IRB for any institution-specific templates and/or requirements regarding the format and content of the consent form.
- Informed Consent Checklist (MS Word, 55K) presents required and additional elements of the consent forms as set forth in Code of Federal Regulations.
- Informed Consent Version Tracker (MS Excel, 20K) provides a template with two examples of tools that sites may use to track informed consent versions; this helps minimize the use of expired versions and the occurrence of consent deviations.
Data Safety and Monitoring Boards
The Data and Safety Monitoring Board (DSMB) is an independent group of experts that advises the NIA Director and the study investigators. The members of the DSMB serve in an individual capacity and provide their expertise and recommendations. The need for DSMB oversight is based on assessment of the study’s overall risk. Investigators may propose a DSMB in their grant application, or NIA may require that a DSMB be established following consideration of review panel’s comments, NIA’s National Advisory Council on Aging (NACA) advice, and/or input from NIA staff.
- Sample Data and Safety Monitoring Board Charter (MS Word, 24K) The DSMB Charter describes the responsibilities of the DSMB to ensure ongoing, independent study review and assure the study is conducted according to the highest scientific and ethical standards.
- DSMB Conflict of Interest and Confidentiality Statement (MS Word, 20K) - All members of the DSMB are required to be independent of the studies being reviewed and need to certify this by signing a DSMB Conflict of Interest and Confidentiality statement.
- DSMB Report - Single Site Open (MS Word, 323K)
- DSMB Report - Single Site Closed (MS Word, 342K)
- DSMB Report - Multi Site Open (MS Word, 449K)
- DSMB Report - Multi Site Closed (MS Word, 348K)
Additional Startup Tools
- Recruitment and Retention Tips (MS Word, 33K) describe approaches to recruitment and retention of older individuals from diverse ethnic and racial groups in clinical research studies.
- Data Management Tips (MS Word, 30K) help to ensure adequate data management processes and procedures in a clinical study. Investigators are encouraged to use Data Management Tips to describe how data will be handled in the study.
- Best Practices for Data Coordinating Centers – This Compendium, developed by the National Heart Lung and Blood Institute (NHLBI) provides helpful tips for clinical researchers and other stakeholders for developing large, multisite clinical trial programs.
Investigators must include in their application proposed adverse event (AE) and serious adverse event (SAE) definitions and discuss their monitoring and reporting. All clinical trials of drugs and biological products conducted under an Investigational New Drug Application (IND) must use definitions of adverse events and adverse reactions and follow the reporting requirements established by 21 Code of Federal Regulations (CFR) Part 312.32. Trials of medical devices conducted under an Investigational Device Exemption (IDE) must use the definitions and reporting requirements established by 21 CFR 812. All other interventional studies must propose their definitions of adverse events and their reporting procedures. See the NIA Guidance on Clinical Trials for additional information .
- Adverse Event Form ( MS Word , 38K or screen-readable PDF , 69K) provides a template for a study form for collecting information about adverse events that is reviewed by safety monitoring bodies.
- Serious Adverse Event Form ( MS Word , 31K or screen-readable PDF , 769K) provides a template for a study form for collecting information about serious adverse events. The form includes major components of the Food and Drug Administration (FDA) Form 3500.
- AE/SAE Process Flow ( MS Word , 79K or screen-readable plain text file , 4K) illustrates how adverse events and serious adverse events are handled within a study.
The NIA Safety Training Course (available below), an online training venue, provides an overview of human subject safety surveillance and reporting requirements in clinical research studies. The intent of the course is to help clinical study investigators and staff understand and implement NIA and regulatory requirements for safe, high quality clinical research. The topics covered include Good Clinical Practice (GCP), Human Subject Protections, Adverse Events and Unanticipated Problems, Safety Monitoring and Reporting Requirements, Safety Monitoring and Oversight: Data and Safety Monitoring Boards (DSMBs) and Safety Officers, Regulatory Requirements and Responsibilities of Principal Investigators, and Data and Safety Monitoring Plans. The course requires about 40 minutes to complete.
Administrative Forms
Site Signature Log - Delegation of Authority Log ( MS Excel, 47K or screen-readable PDF, 294K ) A record of all study personnel and their specific responsibilities, signatures, and dates of involvement during the conduct of a clinical research study.
Note to File Template (MS Word, 20K) - Used by clinical site staff to document protocol deviations or other discrepancies identified during the conduct of the clinical research study and plans for resolution/prevention.
Sample Visit Flow and Schedule (MS Word, 25K) – The visit schedule tracks an individual participant’s progress through the study and helps to ensure that visits take place during the protocol-specified timeframe. The visit flow provides an overview of the activities that take place at each study visit, and may be customized for each study site.
Study Drug/Investigational Product Tracker (MS Excel, 12K) - Used to track study drug/investigational product disposition and accountability by the clinical research site. For multi-site studies under an investigational new drug (IND) application, this tracker could be used by coordinating centers to track the overall distribution of investigational product.
Study Drug/Investigational Product Compliance Log (MS Word, 30K) - Used to track study drug/investigational product disposition and accountability for each individual participant. This form may be used to track protocol adherence via amount dispensed and returned and is designed to be used in conjunction with the Study Drug/Investigational Product Tracker. May also be used to track study drug/investigational return or destruction.
Study-wide Forms
Adverse Events Form ( MS Word, 38K or screen-readable PDF, 68K )
Prior and Concomitant Medications ( MS Word, 34K or screen-readable PDF, 58K )
Protocol Deviations Form ( MS Word, 46K or screen-readable PDF, 80K )
Serious Adverse Events Form ( MS Word, 31K or screen-readable PDF, 769K )
Study Disposition Form ( MS Word, 32K or screen-readable PDF, 56K )
Baseline Visit Forms
Visit Checklist ( MS Word, 34K or screen-readable PDF, 53K )
Eligibility Form ( MS Word, 29K or screen-readable PDF, 184K )
Demographics Form ( MS Word, 32K or screen-readable PDF, 661K )
Medical History Form ( MS Word, 50K or screen-readable PDF, 87K )
Medical History Conventional ( MS Word, 54K or screen-readable PDF,184 K )
Vital Signs Form ( MS Word, 33K or screen-readable PDF, 101K )
Physical Exam Form ( MS Word, 73K or screen-readable PDF, 193K )
Randomization and Enrollment Form ( MS Word, 32K or screen-readable PDF, 806K )
HHS is committed to making its websites and documents accessible to the widest possible audience, including individuals with disabilities. We are in the process of retroactively making some documents accessible. If you need assistance accessing an accessible version of this document, please reach out to the [email protected] .
DISCLAIMER: The contents of this database lack the force and effect of law, except as authorized by law (including Medicare Advantage Rate Announcements and Advance Notices) or as specifically incorporated into a contract. The Department may not cite, use, or rely on any guidance that is not posted on the guidance repository, except to establish historical facts.
- Skip to main content
- Skip to FDA Search
- Skip to in this section menu
- Skip to footer links

The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
U.S. Food and Drug Administration
- Search
- Menu
- Science & Research
- Science and Research Special Topics
- Clinical Trials and Human Subject Protection
FDA's Role: ClinicalTrials.gov Information
Federal law requires responsible parties to register with and submit results information to the ClinicalTrials.gov data bank for certain applicable clinical trials .
Transparency of clinical trial information is important to scientific advancement. Registering certain trials and posting summary results information permits the scientific community to build on the information made available. The public’s participation in clinical trials makes it possible to further advance scientific progress. Posting clinical trial information on ClinicalTrials.gov honors volunteers who participate in research to advance medical science and enhances public trust by creating a transparent and robust public record of clinical trials and information about their results.
Federal law requires:
- A certification of compliance with ClinicalTrials.gov requirements to accompany certain human drug, biological product and device applications and submissions to FDA.
- The inclusion of a particular statement (see below) in the informed consent documents for applicable clinical trials, which are trials that will be entered into the ClinicalTrials.gov databank.
- FDA to oversee compliance and take appropriate enforcement action related to failure to submit required clinical trial information to ClinicalTrials.gov.
Requirements for Certification of Compliance with Certain Product Applications
FDA developed Form FDA 3674 which may be used to provide certification. The certification must accompany certain human drug, biological product and device applications and submissions. In general, FDA recommends that Form FDA 3674 accompany the following applications and submissions to FDA:
- Investigational New Drug Application (IND)
- New Clinical Protocol Submitted to an IND
- New Drug Application (NDA)
- Efficacy Supplement to an Approved NDA
- Biologics License Application (BLA)
- Efficacy Supplement to an Approved BLA
- Abbreviated New Drug Application (ANDA)
- Premarket Approval Application (PMA)
- PMA Panel Track Supplement
- Humanitarian Device Exemption (HDE)
- 510(k) submissions that refer to, relate to or include information on a clinical trial
Federal law does not require the submission of a Form FDA 3674 with an Investigational Device Exemption (IDE) application.
More information is available in FDA Guidance on Form FDA 3674 .
Informed Consent Requirements
Federal law requires the following exact statement to be included in the informed consent documents of applicable clinical trials :
“A description of this clinical trial will be available on http://www.ClinicalTrials.gov , as required by U.S. Law. This Web site will not include information that can identify you. At most, the Web site will include a summary of the results. You can search this Web site at any time.”
See FDA’s guidance on Informed Consent Elements, 21 CFR 50.25(c) for more information.
ClinicalTrials.gov Compliance and Enforcement Activities
The FDA’s compliance activities related to the ClinicalTrials.gov requirements provide the opportunity for responsible parties to take voluntary corrective actions before the agency proceeds with enforcement action. The agency uses a risk-based approach to compliance and enforcement to prioritize the greatest risks to public health.
The final guidance on Civil Money Penalties Relating to the ClinicalTrials.gov Data Bank outlines FDA’s approach to its compliance and enforcement activities related to ClinicalTrials.gov, including potential civil money penalties for violations.
See pre-notices of noncompliance and notices of noncompliance for more information.
Questions Related to ClinicalTrials.gov
See Submit Studies to ClinicalTrials.gov PRS for information about registering clinical trials on ClinicalTrials.gov. Contact [email protected] with questions related to:
- Regulations at 42 CFR Part 11
- How to register studies and how to submit clinical trial results information
- Technical issues related to submission of information to the ClinicalTrials.gov databank
Contact [email protected] with questions about compliance and enforcement of ClinicalTrials.gov requirements.
Complaints to FDA should be reported to the office handling the type of study involved. See FDA contact information for complaints .
Additional resources
- ClinicalTrials.gov – a Three-Part Series
- Final Rule on Clinical Trials Registration and Results Information Submission
- ClinicalTrials.gov - Pre-Notices for Potential Noncompliance
- ClinicalTrials.gov - Notices of Noncompliance and Civil Money Penalty Actions
- ClinicalTrials.gov regulations (42 CFR Part 11)
- Details of the statutory language of Title VIII of FDAAA
- NIH Checklist for evaluating whether a clinical trial is an applicable clinical trial
- Form FDA 3674 (including instructions)
- FDA Guidance on Form FDA 3674
- FDA Guidance on Informed Consent Elements at 21 CFR 50.25(c)
- FDA guidance on Civil Money Penalties Relating to the ClinicalTrials.gov Data Bank

An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.

Clinical trials are medical research studies in which people participate as volunteers. They help researchers better understand the normal biological processes, learn more about diseases and conditions, and develop new treatments and medications.
Find a Clinical Trial
There are several ways to search for clinical trials that may be right for you.
NIAMS Clinical Studies See the full list of featured NIAMS clinical trials taking place at the NIH Clinical Center.
NIH Clinical Studies Find clinical trials sponsored by one or more of NIH’s Institutes and Centers.
ClinicalTrials.gov Search a database of clinical trials available across the country and around the globe. To search for studies accepting healthy volunteers, type in the keywords: 'healthy' and 'normal'.
Resource Information (ClinicalTrials.gov) Find publications related to ClinicalTrials.gov, clinical alerts, and information on subscribing to RSS feeds for new and updated clinical studies.
Participating in a Trial
Once you’ve decided to participate in a trial, learn more about what happens find information to help Get answers to your questions about participating in a clinical trial.
Patient Information Get answers to all of your questions, including the admission process, the NIH campus, and your protections as a volunteer.
Healthy Volunteer Information Find out why healthy volunteers are needed and how you can volunteer to participate in a clinical study.
Glossary of Clinical Trials Terms Learn what all the terms mean so you can better understand clinical trials and make an informed decision about whether to participate.
Clinical Trial Basics
Learn about clinical trials, who conducts them, where they are conducted, why you might participate, and the benefits and risks to participating.
NIH Clinical Research Trials and You Learn what clinical trials are, who participates, questions to ask about the trials, and your protections as a volunteer.
Are Clinical Studies for You? Learn about the risks and benefits of participating in a clinical trial and the questions to discuss with your doctor as you think about whether participating is right for you.
Learn About Clinical Studies Learn the basics about clinical studies, why they are important, and who can participate in a study.
For Health Providers
Referring Physician Information Find strategies and tips for how to refer patients to clinical trials and how to stay involved once your patient is in a trial.

IMAGES
VIDEO
COMMENTS
The Trip Pro offering takes an already wonderful - and free - version of Trip and makes it even better. With more content, more functionality, no adverts and more, it really makes the Trip experience even better ... A smart, fast tool to find high quality clinical research evidence. Searched over 125,000,000 times; Over 70% of clinical ...
What Is A Trip Report In Clinical Research? Trip reports or annotated trip reports are summaries including notes regarding a site visit. The information on these reports is reviewed by lead CRA's and sponsors for review. Follow up letters which are given to sites contain observations made by the monitors based on the contents of the trip reports.
Hamilton S, Bernstein AB, Blakey G, et al. 2016. Developing the Clarity and Openness in Reporting: E3-based (CORE) Reference user manual for creation of clinical study reports in the era of clinical trial transparency. Research integrity and peer review. 1:4. Hamilton S. 2014. Effective authoring of clinical study reports. Medical Writing 23(2).
What Is A Trip Report In Clinical Research?Text "guru" to 31996 to join VIP list!http://www.TheClinicalTrialsGuru.comSite Owner Academy: http://www.theclinic...
Clinical Research Monitoring Findings and Trip Report WritingMy FREE SoCal Meetup: https://www.eventbrite.com/e/the-clinical-trials-guru-all-day-southern-cal...
The focus of Trip's content is high-quality, evidence-based resources such as systematic reviews, clinical guidelines, and evidence-based synopses. These include a broad range of grey literature including regulatory advice, clinical trials, critically appraised topics (CATs), and patient decision aids.
Trip Overview. Trip is a clinical search engine designed to allow users to quickly and easily find a variety of high-quality research evidence to support their practice and/or care. Results provided can be sorted by quality, relevance, date or popularity and organized by the type of evidence via a pyramid graphic under the title of each article.
In this randomized trial involving 10,355 patients with COPD, we compared 52 weeks of a once-daily combination of fluticasone furoate (an inhaled glucocorticoid) at a dose of 100 μg, umeclidinium ...
TRIP (Turning Research into Practice) aims to offer healthcare professionals a complete and updated list of online resources useful for evidence-based practice. TRIP is a database of internet links to information resources selected because they offer free access to high quality content. The TRIP website does not report the criteria used to determine whether the information is high quality. The ...
At Trip, we continuously update our database with thousands of new articles each month, prioritizing the inclusion of high-quality evidence such as guidelines and systematic reviews. Our 'Latest' feature is designed to present these recent additions in a user-centric manner, tailored according to individual profiles. This customization occurs in two primary ways: Clinical area: For…
To complete a clinical trip report. Navigate to the Site Visits screen, then the Clinical Site Visits List view. In the Clinical Site Visits list, drill down on the Visit Start field of the site visit for which you want to complete a trip report. The Trip Report form for the selected site visit appears. Complete or edit fields in the Trip ...
With Vault, you can manage your monitoring trips and information with CTMS Trip Reports. This feature collects all of your trip information into a single Vault object, Monitoring Event, and enables you to automatically format it into a Monitoring Visit Report (MVR) document. Vault uses Trip Report Templates, which your Admin can configure for ...
With many top 20 pharma averaging over 40,000 monitoring trip reports per year, 1 it's not surprising that 25%-30% of total clinical trial costs are attributed to site monitoring. 2. The time spent on monitoring visits is compounded by the reality that many CRAs work across 10+ systems, which are often siloed, adding effort and complexity to ...
The CRA (clinical research associate) is the end user of the Siebel Clinical product. Before visiting a site, the CRA uses the trip report to prepare for the visit. The follow-up items list reminds the CRA of the open activities from previous visits that the CRA can close. After preparing a draft trip report, the CRA makes a hard copy of the ...
For NIH-funded research meeting the definitions of an applicable clinical trial and an NIH-defined Phase III clinical trial, NIH requires reporting of results of valid analyses by sex/gender and race/ethnicity in ClinicalTrials.gov. This policy implements provisions in the 21st Century Cures Act (Public Law 115-255), enacted on December 13, 2016.
The clinical trial process involves protocol development, designing a case record/report form (CRF), and functioning of institutional review boards (IRBs). It also includes data management and the monitoring of clinical trial site activities. The CRF is the most significant document in a clinical study.
Clinical Trial Monitoring Reports and How to Write Them. Oct 31. Among the most important aspects of study is observation. Overseeing the advancement of any stage, measure, process, and procedure in real time is essential to the accurate conclusion of any clinical trial undertaking. Normal monitoring actions are needed to guarantee caliber ...
About Administering and Using Clinical Trip Reports. The study managers or clinical administrators can set up templates for trip reports. The CRAs (clinical research associates) then use these templates when they create trip reports to record their visits to sites. Advantages of using the trip reports in Siebel Clinical are:
Temporal trends and characteristics associated with racial, ethnic, and sex representation in COVID-19 clinical trials: A systematic review and meta-analysis, Contemporary Clinical Trials, 143 ...
ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. Explore 497,446 research studies in all 50 states and in 222 countries. See listed clinical studies related to the coronavirus disease (COVID-19)
The REPORT guide is a 'How to' guide to help you report your clinical research in an effective and transparent way. It is intended to supplement established first choice reporting tools, such as Consolidated Standards of Reporting Trials (CONSORT), by adding tacit knowledge (ie, learnt, informal or implicit knowledge) about reporting topics that we have struggled with as authors or see ...
Glucagon-like peptide 1 (GLP-1) agonists are medications approved for treatment of diabetes that recently have also been used off label for weight loss. 1 Studies have found increased risks of gastrointestinal adverse events (biliary disease, 2 pancreatitis, 3 bowel obstruction, 4 and gastroparesis 5) in patients with diabetes. 2-5 Because such patients have higher baseline risk for ...
Clinical trials are voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of ...
With Vault, you can manage your users' monitoring trips and information with CTMS Trip Reports. This feature collects all trip information into a single Vault object, Monitoring Event, and enables users to automatically format it into a Monitoring Visit Report (MVR) document. Vault uses formatted output to create documents for your Monitoring ...
An important component of preparing is to look at past clinical monitoring reports and audit reports, if any have been conducted. If a previous site interim visit reported a problem, that requires follow-up during the next visit. Time on-site is limited, so these issues should be prioritized. A risk-based approach to monitoring will also ...
Back to top. Forms and Templates Administrative Forms. Site Signature Log - Delegation of Authority Log ( MS Excel, 47K or screen-readable PDF, 294K) A record of all study personnel and their specific responsibilities, signatures, and dates of involvement during the conduct of a clinical research study. Note to File Template (MS Word, 20K) - Used by clinical site staff to document protocol ...
Learn about the FDA's compliance and enforcement responsibilities for ClinicalTrials.gov, ensuring proper conduct of clinical trials.
Clinical research is undergoing a paradigm shift from a siloed disease approach to the recognition of cardiorenal-metabolic multiorgan conditions in view of the crosstalk among the heart, kidneys, liver, intestinal microbiome, and brain. ... Disclosures Dr Zannad reports personal fees from 89bio, Applied Therapeutics, Bayer, Betagenon ...
Get Information for the Public, Researchers, and Advocates Related to NIA Clinical Trials This page serves as a hub for information on NIA-conducted and -supported clinical trials, health information on participating in a clinical trial, and information on finding and joining a clinical trial. For the Public For Advocates, Journalists, & Stakeholders For Researchers
Clinical Trial Basics. Learn about clinical trials, who conducts them, where they are conducted, why you might participate, and the benefits and risks to participating. NIH Clinical Research Trials and You Learn what clinical trials are, who participates, questions to ask about the trials, and your protections as a volunteer.