A .gov website belongs to an official government organization in the United States.
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Symptoms and Complications
- How It Spreads
- Travel and Living Abroad
- Areas at Risk for Zika
- Show All Home
- Clinical Signs and Symptoms
- Testing and Diagnosis
- Treatment and Prevention
- Sexual Transmission
- Clinical Considerations for Pregnant Persons
- Transmission
- Information for HCT/Ps Establishments
- Mosquito Control
- Vector-Borne Diseases


For Professionals
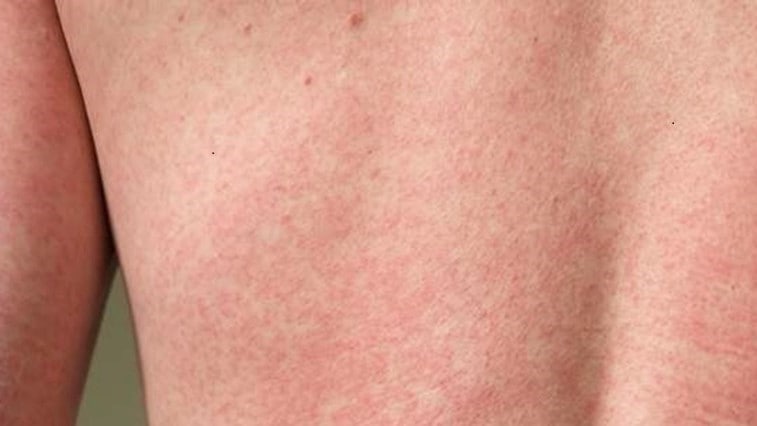
Zika virus is primarily spread by mosquitoes and can cause serious birth defects. Learn about areas at risk, the illness it causes, and ways to prevent it.
For Everyone
Health care providers, public health.
- Skip to main content
- Skip to FDA Search
- Skip to in this section menu
- Skip to footer links

The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
U.S. Food and Drug Administration
- Search
- Menu
- Emergency Preparedness and Response
- Counterterrorism and Emerging Threats
- Medical Countermeasures Initiative (MCMi)
Zika Virus Response Updates from FDA

Fast Facts : About Zika | Locations Affected | Guillain-Barré Syndrome | Pregnancy | Medical Products | Prevention
Zika Information from FDA : Updates by Date | Safety of the Blood Supply | Emergency Use Authorization | Investigational Products | Fraudulent Products | Using Insect Repellants Safely | Events | More About FDA’s Role | Contact FDA | Related Links | Resources for Healthcare Providers | Translations (Spanish, Portuguese)
Zika virus is spread to people primarily through the bite of an infected Aedes species mosquito. Most people never know that they have been infected with the virus. It is estimated that four out of five people with Zika virus infections have no symptoms at all. When symptoms do occur, the most common symptoms are fever, rash, joint pain, and conjunctivitis (red eyes). Even in those who develop symptoms, the illness is usually mild, with symptoms lasting from several days to a week.

A pregnant woman applies mosquito repellant. Using insect repellants will help to protect her from being bitten by a mosquito that may be carrying a virus such as Zika; this will also protect her unborn baby from the virus. (Image: CDC/Division of Vector-borne Diseases)
Locations Affected
Prior to 2015, Zika virus outbreaks had occurred in areas of Africa, Southeast Asia, and the Pacific Islands. However, in May 2015, the Pan American Health Organization (PAHO) issued an alert (PDF, 199 KB) regarding the first confirmed Zika virus infection in Brazil. For information on current outbreaks, see from CDC:
- Zika in the US
- Zika Travel Information
Guillain-Barré Syndrome
Since the outbreak in Brazil began, we have seen reports of Guillain-Barré syndrome (a disorder in which the immune system attacks the nervous system) and birth defects. More: Zika and Guillain-Barré Syndrome , from CDC
Zika virus can be transmitted from a pregnant mother to her fetus. Scientists at the Centers for Disease Control and Prevention (CDC) concluded , after careful review of existing evidence, that Zika virus is a cause of microcephaly , a condition in which a baby’s brain and head is smaller than expected, and other severe fetal brain defects. In the April 13, 2016 report published in the New England Journal of Medicine , the CDC authors describe a rigorous weighing of evidence using established scientific criteria.
The finding that Zika virus infection can cause microcephaly and other severe fetal brain defects means that a woman who is infected with Zika during pregnancy has an increased risk of having a baby with these health problems. It does not mean, however, that all women who have Zika virus infection during pregnancy will have babies with problems. As has been seen during the current Zika outbreak, some infected women have delivered babies that appear to be healthy. More: Zika and pregnancy, from CDC , and CDC updates guidance for infants born to mothers with possible Zika virus infection during pregnancy (October 19, 2017)
Preventing pregnancy: If you decide that now is not the right time to have a baby, talk to your healthcare provider. View information on the safety and effectiveness of FDA-approved medicines and devices for birth control (en Español Guía de Métodos Anticonceptivos (PDF, 433 KB))
Medical Products
There are no FDA-approved vaccines for Zika virus. Several investigational vaccines are under development, including early human clinical trials .
There are no FDA-approved treatments for Zika virus , nor is the FDA aware of treatments in advanced development for Zika at this time. Also see Zika Virus Treatment Research from NIAID
Diagnostics: FDA-authorized diagnostic tests for detecting Zika virus antibodies:
- ZIKV Detect 2.0 IgM Capture ELISA - On May 23, 2019, FDA authorized marketing of the ZIKV Detect 2.0 IgM Capture ELISA to detect Zika virus immunoglobulin (IgM) antibodies in human blood. The ZIKV Detect 2.0 IgM Capture ELISA is the first Zika diagnostic test the FDA has allowed to be marketed in the U.S. FDA reviewed the data for the test through the De Novo premarket review pathway. Previously, tests for detecting Zika virus immunoglobulin (IgM) antibodies—including the ZIKV Detect 2.0 IgM Capture ELISA—had been authorized only for emergency use under the FDA’s Emergency Use Authorization (EUA) authority. For more information, see Serological assays below
- ADVIA Centaur Zika test – On July 17, 2019, FDA cleared the ADVIA Centaur Zika test. This is the second Zika diagnostic test FDA has allowed to be marketed in the U.S. for detecting Zika virus IgM antibodies. Previously, the test had been authorized only for emergency use under FDA’s EUA authority.
- LIAISON XL Zika Capture IgM Assay II – On October 28, 2019, FDA cleared the LIAISON XL Zika Capture IgM Assay II for detecting Zika virus IgM antibodies. Previously, the test had been authorized only for emergency use under FDA’s EUA authority.
- DPP Zika IgM Assay System – On June 3, 2020, FDA cleared a similar DPP Zika IgM System for detecting Zika virus IgM antibodies. Previously, the test had been authorized only for emergency use under FDA’s EUA authority.
FDA encourages commercial diagnostic developers and researchers developing laboratory developed tests for Zika virus to submit an EUA request or consider pursuing a premarket submission. FDA will work interactively with developers to support such requests. See Zika Virus Emergency Use Authorization for information about Zika virus diagnostics available under EUA.
FDA stands ready to work with medical product developers to clarify regulatory and data requirements necessary to move products forward in development as quickly as possible.
See also: Zika Symptoms, Diagnosis, & Treatment, from CDC
The best way to prevent Zika and other diseases spread by mosquitoes is to avoid being bitten. More: Prevention, from CDC
Zika Information from FDA
Updates by date, latest updates.
- May 20, 2024: Information for Human Cell, Tissue, and Cellular and Tissue-Based Product (HCT/P) Establishments Regarding FDA’s Determination that Zika Virus is no Longer a Relevant Communicable Disease Agent or Disease (RCDAD) - Because Zika virus (ZIKV) is no longer an RCDAD, HCT/P establishments may discontinue screening donors for ZIKV and revise their relevant procedures to reflect this change.
- March 9, 2023: FDA held a Grand Rounds lecture: Microphysiological Systems as Novel Disease Models and Drug Development Tools . This presentation by researchers from FDA's National Center for Toxicological Research (NCTR) evaluates nonhuman primate testicular organoids for use as an in vitro model of Zika virus infection. A recording is available.
Additional updates (2021 and earlier)
May 12, 2021: Information for Blood Establishments Regarding FDA’s Determination that Zika Virus is no Longer a Relevant Transfusion-Transmitted Infection , and Withdrawal of Guidance titled “Revised Recommendations for Reducing the Risk of Zika Virus Transmission by Blood and Blood Components” - FDA has determined Zika virus (ZIKV) is no longer an RTTI under FDA’s regulations because, as discussed further in the guidance, the available evidence demonstrates that ZIKV no longer has sufficient incidence and/or prevalence to affect the potential donor population. Accordingly, FDA withdrew the guidance titled, “Revised Recommendations for Reducing the Risk of Zika Virus Transmission by Blood and Blood Components,” dated July 2018.
- June 3, 2020: FDA cleared the DPP Zika IgM System for detecting Zika virus IgM antibodies. Previously, the test had been authorized only for emergency use under FDA’s EUA authority.
November 25, 2019: Publication - FDA Zika virus reference panel for molecular-based diagnostic devices supports product testing for Emergency Use Authorization and 510(k) submissions - read the full publication in The Journal of Molecular Diagnostics
October 28, 2019: FDA cleared the LIAISON XL Zika Capture IgM Assay II for the presumptive qualitative detection of Zika virus IgM antibodies in human sera collected from individuals meeting the CDC Zika virus clinical and/or epidemiological criteria. Previously, the test had been authorized only for emergency use under FDA’s EUA authority. FDA revoked the EUA for the LIAISON XL Zika Capture IgM Assay II test, initially issued on April 5, 2017.
July 17, 2019: FDA cleared the ADVIA Centaur Zika test. This is the second Zika diagnostic test FDA has allowed to be marketed in the U.S. for detecting Zika virus IgM antibodies. Previously, the test had been authorized only for emergency use under FDA’s EUA authority. FDA revoked the EUA for the ADVIA Centaur Zika test, initially issued on September 18, 2017.
July 3, 2019: In a letter to FDA dated June 18, 2019, Luminex Corporation requested that the EUA for the xMAP MultiFLEX Zika RNA Assay issued on August 4, 2016, and amended on January 7, 2017, and May 19, 2017, be withdrawn. Luminex has decided to discontinue manufacture of the product and there is no remaining viable inventory of the xMAP MultiFLEX Zika RNA Assay. As a result, this product will no longer be marketed, and these circumstances make revocation appropriate to protect the public health or safety. Accordingly, on July 3, 2019, FDA revoked the EUA for xMAP MultiFLEX Zika RNA Assay, pursuant to section 564(g)(2) of the Act. As of July 3, 2019, the xMAP MultiFLEX Zika RNA Assay that was authorized by FDA for use by clinical laboratories for the qualitative detection of RNA from Zika virus is no longer authorized by FDA.
May 23, 2019: FDA authorizes marketing of first diagnostic test for detecting Zika virus antibodies - FDA authorized marketing (PDF, 175 KB) of the ZIKV Detect 2.0 IgM Capture ELISA to detect Zika virus immunoglobulin (IgM) antibodies in human blood. FDA reviewed data for the ZIKV Detect 2.0 IgM Capture ELISA test through the De Novo premarket review pathway. Also see Emergency Use Authorization below
April 18, 2019: EUA amendment - In response to Siemens Healthcare Diagnostic Inc.’s request, FDA concurred (PDF, 137 KB) with the request to modify the ADVIA Centaur Zika test to include surfactant in the ADVIA Centaur Zika IgM assay reagent buffers and the related updates of the Instructions for Use (PDF, 2.8 MB). For more information, see Emergency Use Authorizations (Devices)
February 28, 2019: Important Information for Human Cell, Tissue, and Cellular and Tissue-Based Product (HCT/P) Establishments Regarding Zika Virus Transmission Risk in the World [ARCHIVED] - CDC has changed information on its Blood and Tissue Safety webpage used to communicate epidemiological information about Zika virus (ZIKV) to the blood and tissue collection community. The webpage includes a world map of areas with risk of Zika for other countries and territories outside of U.S. states. A new process has been developed to indicate risk for these areas that assigns one of four categories. FDA considers countries and territories outside the U.S. states categorized as “Red” (current outbreak) or “Purple” (any prior or current reports of mosquito-borne Zika transmission) as areas with increased risk of ZIKV transmission.
For updates by date before 2019, please visit our archive .
Safety of the Blood Supply
FDA is responsible for regulatory oversight of the U.S. blood supply. FDA works closely with other parts of the Public Health Service (PHS) to establish blood standards, and to identify and respond to potential threats to blood safety or supply.
Zika updates - safety of the blood supply
- On February 28, 2019, FDA published a web page: Important Information for Human Cell, Tissue, and Cellular and Tissue-Based Product (HCT/P) Establishments Regarding Zika Virus Transmission Risk in the World (archived) - CDC has changed information on its Blood and Tissue Safety webpage used to communicate epidemiological information about ZIKV to the blood and tissue collection community. The webpage includes a world map of areas with risk of Zika for other countries and territories outside of U.S. states. A new process has been developed to indicate risk for these areas that assigns one of four categories. FDA considers countries and territories outside the U.S. states categorized as “Red” (current outbreak) or “Purple” (any prior or current reports of mosquito-borne Zika transmission) as areas with increased risk of ZIKV transmission.
Revised guidance - On July 6, 2018, FDA announced the availability of a revised final guidance: Revised Recommendations for Reducing the Risk of Zika Virus Transmission by Blood and Blood Components . This revised guidance replaces the August 2016 guidance, which recommended universal nucleic acid testing for Zika virus of individual units of blood donated in the U.S. states and territories. The revised guidance explains that, in order to comply with applicable testing regulations, blood establishments must continue to test all donated Whole Blood and blood components for Zika virus using a nucleic acid test. The revised guidance explains the basis for the FDA’s determination that pooled testing of donations using a screening test licensed for such use by the FDA is a sufficient method for complying with these regulations and effectively reducing the risk of Zika Virus transmission, unless there is an increased risk of local mosquito-borne transmission of Zika virus in a specific geographic area that would trigger individual donation testing in that location. Alternatively, blood establishments may use an FDA-approved pathogen-reduction device for plasma and certain platelet products. ( Federal Register notice ) Also see: FDA announces revised guidance on the testing of donated blood and blood components for Zika virus
Revised guidance - On May 2, 2018, FDA issued revised guidance for establishments that make donor eligibility determinations for donors of human cells, tissues, and cellular and tissue-based products (HCT/Ps): Donor Screening Recommendations to Reduce the Risk of Transmission of Zika Virus by Human Cells, Tissues, and Cellular and Tissue-Based Products; Guidance for Industry (PDF, 86 KB). This guidance updates information in the March 2016 guidance by: This update supports the continuation of recommendations to screen living donors of HCT/Ps for risks of infection with ZIKV based on geographic areas with risk.
This update supports the continuation of recommendations to screen living donors of HCT/Ps for risks of infection with ZIKV based on geographic areas with risk. Previously, on March 1, 2016, as an additional safety measure against the emerging Zika virus outbreak, FDA issued this guidance as a part of ongoing efforts to protect HCT/Ps and blood products from Zika virus transmission. Read the news release
providing findings from more recent epidemiological studies including impact on public health;
reporting new data that informs the potential for transmission of ZIKV;
discussing the current status of availability of ZIKV tests;
updating sexual contact risk factors;
updating when an area is considered to have an increased risk for ZIKV transmission; and,
providing additional scientific references.
On July 5, 2018, FDA approved the Procleix Zika Virus Assay, manufactured by Grifols Diagnostics Solutions, Inc. The Procleix Zika Virus Assay is a qualitative nucleic acid test for the detection of Zika virus RNA in individual plasma specimens obtained from volunteer donors of whole blood and blood components for transfusion. It is also intended for use in testing plasma or serum specimens to screen other living donors of organs and human cells, tissues, and cellular and tissue-based products (HCT/Ps), and in testing blood specimens to screen cadaveric donors. The assay is intended for use in testing individual donor samples. It is also intended for use in testing pools of human plasma composed of equal aliquots of not more than 16 individual specimens from volunteer donors of whole blood components. It is not intended for use as an aid in the diagnosis of Zika virus infection. For more information see the approval letter (PDF, 41.2 KB) and Safety of the Blood Supply below
On October 5, 2017, FDA approved the first test for screening Zika virus in blood donations . FDA approved the cobas Zika test, a qualitative nucleic acid test for the detection of Zika virus RNA in individual plasma specimens obtained from volunteer donors of whole blood and blood components, and from living organ donors. It is intended for use by blood collection establishments to detect Zika virus in blood donations, not for the individual diagnosis of Zika virus infection. Before October 5, 2017, several blood collection establishments used the cobas Zika test under IND in order to follow the recommendations in the FDA’s 2016 guidance document. The data collected from this testing, and from additional studies performed by the manufacturer, demonstrated that the cobas Zika test is an effective test to screen blood donors for Zika virus infection. The test’s clinical specificity was evaluated by testing individual samples from blood donations at five external laboratory sites, resulting in clinical specificity of more than 99 percent. The cobas Zika test is intended for use on the fully automated cobas 6800 and cobas 8800 systems. The cobas Zika test, cobas 6800, and cobas 8800 systems are manufactured by Roche Molecular Systems, Inc. Previously, on March 30, 2016, FDA announced the availability of an investigational test to screen blood donations for Zika virus. The screening test may be used under an investigational new drug application (IND) for screening donated blood in areas with active mosquito-borne transmission of Zika virus. Once screening of blood donations for Zika virus using the investigational test begins, blood establishments in Puerto Rico may resume collecting donations of Whole Blood and blood components.
FDA continues to work with public health authorities in territories with confirmed Zika virus to take rapid and appropriate steps to help ensure safe blood is available. Prior to the revised guidance issued on August 26, 2016, FDA took steps to protect the blood supply in areas with confirmed Zika virus transmission.
On March 5, 2016, the first batch of blood products arrived in Puerto Rico in response to HHS efforts to arrange and fund shipment of blood from the continental United States to Puerto Rico to ensure an adequate supply of safe blood for island residents. The Commonwealth of Puerto Rico was the first U.S. territory to experience active mosquito-borne Zika transmission.of an investigational test to screen blood donations for Zika virus. The screening test may be used under an investigational new drug application (IND) for screening donated blood in areas with active mosquito-borne transmission of Zika virus. Once screening of blood donations for Zika virus using the investigational test begins, blood establishments in Puerto Rico may resume collecting donations of Whole Blood and blood components.
On March 13, 2017, the CDC announced that based on a retrospective analysis of Zika virus (ZIKV) infections they identified a potential increased risk to blood and tissue safety, including semen, in Florida’s Miami-Dade, Palm Beach, and Broward counties dating back to June 15, 2016. While Miami-Dade County is the only part of Florida currently (July 29, 2016 to present) designated by CDC as an area of active ZIKV transmission for the purposes of blood and tissue safety intervention, people in this part of Florida regularly travel within and between these three counties and may not recognize that they have been in an area of active ZIKV transmission. This information has been added to CDC’s webpage used to communicate epidemiological information about ZIKV to the blood and tissue collection community.
The potential increased risk to blood and tissue safety, and particularly to semen, in this area due to CDC’s announcement is considered to be very low. However, as a precaution, the Food and Drug Administration is informing establishments that collect tissues (i.e., human cell, tissues, and cellular and tissue-based products – HCT/Ps) and blood components of the potential increased risk, so they may consider whether and how this new information impacts their practices.
Also see the FDA’s communication to tissue establishments: Important Information for Human Cell, Tissue and Cellular and Tissue-based Product (HCT/P) Establishments Regarding Zika Virus
Emergency Use Authorization
FDA stands ready to use our authorities to the fullest extent to help facilitate the development and availability of products for Zika virus. Under the FDA’s Emergency Use Authorization (EUA) mechanism, the agency can enable the use of an unapproved medical product, or the unapproved use of an approved medical product, during emergencies, when, among other circumstances, there are no adequate approved, and available alternatives. An EUA is an important mechanism that allows broader access to available medical products under specific circumstances.
Zika EUA information
- While many people with Zika virus infection experience no symptoms, the virus can pose potentially serious risks to the public health. Access to a diagnostic test that can identify patients with Zika virus infections is critical to supporting response efforts and expanding domestic readiness. Potential links between Zika virus infection and neurological complications (i.e., Guillain-Barré Syndrome), as well as microcephaly and other poor pregnancy outcomes associated with Zika virus infection during pregnancy, have also increased the importance of having a diagnostic test available for Zika virus. As there are no commercially available diagnostic tests cleared or approved by the FDA for the detection of Zika virus infection, it was determined that an EUA is crucial to ensure timely access to a diagnostic tool.
- An EUA is a tool that FDA can use to allow the use of certain medical products for emergencies based on scientific data. The U.S. Secretary of Health and Human Services (HHS) has declared that circumstances exist to allow the emergency use of authorized diagnostic tests for Zika virus infection.
- Draft EUA review templates for Zika are available to product sponsors/manufacturers by email request to: [email protected]
Zika diagnostic tests currently authorized under EUA
Performance characteristics of zika virus diagnostic tests.
FDA has posted tables detailing performance characteristics of Zika virus diagnostic tests (assays) currently available for use under EUA. The tables include information about analytical sensitivity, along with other performance characteristics determined during EUA evaluation. (May 3, 2018)
- Table 1: Molecular ZIKV EUA Assays - Performance Characteristics (PDF, 200 KB)
- Table 2: Molecular ZIKV EUA Assays - Key Characteristics (PDF, 247 KB)
Tests currently authorized under EUA are listed on the CDRH page, Emergency Use Authorizations for Medical Devices .
For a list of FDA-authorized diagnostic tests for detecting Zika virus antibodies, see Diagnostics above.
Nucleic acid testing-based assays (molecular tests) - detect genetic material in samples of bodily fluids, such as serum and urine, to diagnose active Zika infection
Serological assays - detect antibodies against Zika virus in the blood, to assess whether individuals who may have recently been exposed to Zika have actually been infected
Also see the August 17, 2017 press release: FDA provides new tools for the development and proper evaluation of tests for detecting Zika virus infection - As an additional measure in the fight against Zika virus, FDA made available a panel of human plasma samples to aid in the regulatory evaluation of serological tests, to help ensure that tests to detect recent Zika infection are accurate and reliable, and to help test manufacturers know if their tests differentiate between infections with Zika virus or other flaviviruses such as Dengue, and West Nile viruses, which all have similar antibodies. More: Zika Virus Reference Materials and FDA Zika virus reference panel for molecular-based diagnostic devices supports product testing for Emergency Use Authorization and 510(k) submissions
Investigational Products
Medical Products | Genetically Engineered Mosquitoes
Medical Products (Vaccines, Therapeutics, Diagnostics)
Vaccines and therapeutics: There are no FDA-approved vaccines or treatments for Zika at this time. Several investigational vaccines are under development, including early human clinical trials . FDA is prepared to evaluate the safety and efficacy of any investigational vaccines and therapeutics that might be developed to help mitigate this outbreak.
Diagnostics: For a list of FDA-authorized diagnostic tests for detecting Zika virus antibodies, see Diagnostics above.
FDA encourages commercial diagnostic developers and researchers developing laboratory developed tests for Zika virus to submit an EUA request or consider pursuing a premarket submission. FDA will work interactively with developers to support such requests. See Zika Virus Diagnostic Development for information on FDA support for Zika virus diagnostic development and Emergency Use Authorization for information about Zika virus diagnostics available under EUA.
To help Zika diagnostic manufacturers assess traceability of their tests (a requirement for Emergency Use Authorization), FDA has created the FDA Zika Virus Reference Materials for NAT-based IVD devices , available upon request to Zika device developers who have a pre-EUA submission with the agency and have established the analytical and clinical performance of their assay. In July 2017, FDA also made available a panel of human plasma samples to aid in the regulatory evaluation of serological tests to detect recent Zika virus infection. Developers planning a future premarket submission will have priority to receive the panel of human plasma samples, considering the grant of a De Novo classification request for the ZIKV Detect 2.0 IgM Capture ELISA on May 23, 2019. View an infographic about the FDA Zika Virus Reference Materials (PDF, 120 KB)
Genetically engineered mosquitoes related to Zika response
Final guidance - October 4, 2017: FDA Issues Final Guidance Clarifying FDA and EPA Jurisdiction over Mosquito-Related Products [ARCHIVED] - The final Guidance for Industry #236 – Clarification of FDA and EPA Jurisdiction over Mosquito-Related Products (PDF, 85 KB) – clarifies that mosquito-related products intended to function as pesticides by preventing, destroying, repelling, or mitigating mosquitoes for population control purposes, and that are not intended to cure, mitigate, treat, or prevent a disease are not “drugs” under the Federal Food, Drug, & Cosmetic Act, and will be regulated by the EPA under the Federal Insecticide, Fungicide, and Rodenticide Act. The FDA will continue to have jurisdiction over mosquito-related products that are intended to prevent, treat, mitigate, or cure a disease (including by an intent to reduce the level, replication, or transmissibility of a pathogen in mosquitoes). ( Federal Register notice )
The Zika virus outbreak highlights the importance that novel vector control measures may play in protecting the public health. Reviewing the use of innovative strategies to help suppress the population of virus-carrying mosquitoes is one of many activities in which FDA is engaged to help mitigate the threat of vector-borne epidemics, such as Zika.
FDA’s Center for Veterinary Medicine reviewed information in an Investigational New Animal Drug (INAD) file from Oxitec, Ltd., regarding the company’s genetically engineered line of the mosquito Aedes aegypti (OX513A), with the intent of suppressing the population of that mosquito at the release site(s). Ae. aegypti is known to transmit the debilitating human virus-caused diseases Zika, dengue, yellow fever, and chikungunya. More: Oxitec Mosquito
On March 11, 2016, in compliance with FDA regulations, FDA released for public comment a draft environmental assessment (EA) (PDF, 33 MB) submitted by Oxitec, Ltd., that assesses the potential environmental impacts of a field trial of the company’s genetically engineered (GE) Aedes aegypti mosquitoes (OX513A) in Key Haven, Florida. Ae. aegypti is known to transmit potentially debilitating human viral diseases, including Zika, dengue, yellow fever and chikungunya. The FDA also released a preliminary finding of no significant impact (FONSI) (PDF, 148 KB) that agrees with the draft EA’s conclusion that the field trial of such GE mosquitoes will not result in significant impacts on the environment.
The goal of the proposed field trial is to determine whether released Oxitec GE mosquitoes will mate with local wild-type Aedes aegypti and suppress their population at the release site. The proposed study is not seeking to evaluate whether release of Oxitec’s GE mosquitoes will reduce Zika virus transmission. Oxitec’s mosquitoes are one possible approach that could be incorporated into an integrated program to help mitigate the threat of vector-borne epidemics; however, it is too early to say with any certainty whether such an approach would be successful.
The public comment period for the draft Environmental Assessment and preliminary Finding of No Significant Impact concerning investigational use of Oxitec OX513A mosquitoes closed on May 13, 2016. Because this is a first of its kind application, FDA understands how important the public comment period process is.
August 5, 2016: FDA Releases Final Environmental Assessment for Genetically Engineered Mosquito [ARCHIVED] - FDA has completed the environmental review for a proposed field trial to determine whether the release of Oxitec Ltd.’s genetically engineered (GE) mosquitoes (OX513A) will suppress the local Aedes aegypti mosquito population in the release area at Key Haven, Florida. After considering thousands of public comments, FDA has published a final environmental assessment (EA) (PDF, 3 MB) and finding of no significant impact (FONSI) (PDF, 198 KB) that agrees with the EA’s conclusion that the proposed field trial will not have significant impacts on the environment. FDA’s finalization of the EA and FONSI does not mean that Oxitec’s GE mosquitos are approved for commercial use. Oxitec is responsible for ensuring all other local, state, and federal requirements are met before conducting the proposed field trial, and, together with its local partner, the Florida Keys Mosquito Control District, to determine whether and when to begin the proposed field trial in Key Haven, Florida.
January 18, 2017: FDA Requests Comments on Documents Related to Certain Biotechnology and Mosquito-related Products - FDA is requesting public comment on a draft revised guidance (PDF, 200 KB) on the regulation of animals with intentionally altered genomic DNA, including animals produced through the use of genome editing and genetic engineering, and a draft guidance (PDF, 74 KB) that clarifies which mosquito-related products FDA regulates and which such products EPA regulates, regardless of whether these mosquito-related products are developed using biotechnology. Also see Oxitec Mosquito ; Q&A on FDA Regulation of Intentionally Altered Genomic DNA in Animals ; and FDA Voice: FDA’s Science-based Approach to Genome Edited Products
April 12, 2017: FDA is extending the comment period to continue seeking public input on draft revised guidance for industry #187 - Regulation of Intentionally Altered Genomic DNA in Animals (PDF, 200 KB). The FDA is taking this action in response to requests for additional time to submit comments. The comment period will now close on June 19, 2017 . Also see: FDA Requests Comments on Documents Related to Certain Biotechnology and Mosquito-related Products and Q&A on FDA Regulation of Intentionally Altered Genomic DNA in Animals
October 4, 2017: FDA Issues Final Guidance Clarifying FDA and EPA Jurisdiction over Mosquito-Related Products - The final Guidance for Industry #236 – Clarification of FDA and EPA Jurisdiction over Mosquito-Related Products (PDF, 85 KB) (additional details above)
Fraudulent Products
- Unfortunately, during outbreak situations, fraudulent products claiming to prevent, treat or cure a disease almost always appear. FDA monitors for fraudulent products and false product claims related to the Zika virus and takes appropriate action to protect consumers. Consumers who have seen these fraudulent products or false claims are encouraged to report them to the FDA.
Using Insect Repellents Safely
- All insect repellents, including products combined with sunscreen, should be used according to instructions on the label.
- Use insect repellents that contain active ingredients registered by the Environmental Protection Agency (EPA) for use on skin and clothing. EPA registration of insect repellent active ingredients indicates the materials have been reviewed and approved for human safety and effectiveness when applied according to instructions on the label.
- Don't use insect repellent on babies. Repellent used on older children should contain no more than 10 percent DEET. Oil of eucalyptus products should not be used in children under 3 years.
For a list of events 2019 and earlier, please visit our archive .
More About FDA's Role
FDA is committed to working with the global community as it responds to the Zika virus outbreak. The FDA has a critical role in facilitating the development, and availability of investigational products for use against emerging infectious diseases, such as the Zika virus.
FDA is actively working with our Federal colleagues at the CDC, National Institutes of Health (NIH), and the Biomedical Advanced Research and Development Authority (BARDA), and is prepared to evaluate the safety and efficacy of any investigational vaccines and therapeutics that might be developed to help mitigate this outbreak. The agency is also encouraging development of diagnostic tests that may be useful for identifying the presence of the virus, and is taking steps to help ensure the safety of our nation’s blood supply.
While FDA cannot comment on the development of specific medical products, it’s important to note that every FDA regulatory decision is based on a risk-benefit assessment of scientific data that includes the context of use for the product and the patient population being studied. Approaches that will be able to show whether a product has a favorable risk-benefit profile for its proposed use may require careful planning. This may prove challenging for Zika virus since its symptoms are often mild or nonspecific.
Emergency use: FDA stands ready to use our authorities to the fullest extent to help facilitate the development and availability of products for Zika virus, as we did during the 2014 Ebola epidemic. Under the FDA’s Emergency Use Authorization (EUA) mechanism, the agency can enable the use of an unapproved medical product, or the unapproved use of an approved medical product, during emergencies, when, among other circumstances, there are no adequate approved, and available alternatives. An EUA is an important mechanism that allows broader access to available medical products under specific circumstances.
Blood supply: FDA is responsible for regulatory oversight of the U.S. blood supply. FDA works closely with other parts of the Public Health Service (PHS) to establish blood standards, and to identify and respond to potential threats to blood safety or supply. More: Keeping Blood Transfusions Safe: FDA's Multi-layered Protections for Donated Blood
Translations
Español Português
Note: Spanish and Portuguese translations of this page are archived, and were last updated on the date listed at the bottom of the archived page.
Contact FDA
General Info/Consumers 1-888-INFO-FDA / (1-888-463-6332)
Report a Fraudulent Zika Product Report form and instructions
Press Office of Media Affairs [email protected] 301-796-4540
Clinicians Emergency Investigational New Drug (EIND) Applications for Antiviral Products
Diagnostic Product Sponsors/Manufacturers - EUA Templates Draft EUA review templates for Zika are available by email request to: [email protected]

Related Links
- Zika Virus Information from CDC
- Zika Virus Health Information Resources (National Library of Medicine)
- How to Avoid Bug Bites (CDC)
- About Emergency Use Authorization
- The FDA's Drug Review Process: Ensuring Drugs Are Safe and Effective
Resources for Healthcare Providers
- Zika Information for Health Care Providers (CDC)
- Zika Training for Health Care Providers (CDC)
- When to test for Zika virus (CDC) (PDF, 332 KB)
- Promoting Stress Management for Pregnant Women during the Zika Virus Disease Outbreak: A Resource for Healthcare Providers, and Planning Resources (HHS)
- Guidance for U.S. Laboratories Testing for Zika Virus Infection (CDC)
Sign up to receive email alerts on emergency preparedness and response topics from FDA, including medical countermeasures and emerging infectious diseases.

European Centre for Disease Prevention and Control
An agency of the European Union
- Infectious disease topics
- Zika virus infection
- Threats and outbreaks
- Zika transmission
Zika transmission Archived
Zika transmission maps have stopped being updated in January 2018, as a result as a result of the significant slow-down of the epidemic. ECDC is working with the World Health Organization and the United States CDC to assess the current risk of Zika virus transmission in countries and territories globally
Information on the level of Zika transmission is useful for public health professionals to evaluate the level of risk for people who may be planning to travel to or are recently returning from areas with possible local transmission.
A revised scheme has been developed by WHO, in collaboration with the US CDC and ECDC, to categorise the epidemiological profile of vector-borne Zika virus transmission in countries and territories. The classification can be applied at the national level as well as subnational level when the necessary information is available. ECDC has adapted the classification scheme to reflect the risk to travellers more accurately. It replaces the previous ECDC classification and the WHO interim guidance on Zika virus surveillance published in April 2016.
For a summary description of the revised scheme and a description of the ECDC adaptation see the updated ECDC rapid risk assessment . For the full description of the WHO categories, see the Zika virus country classification scheme , Interim guidance, March 2017.
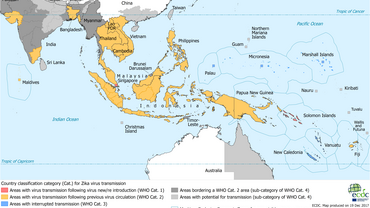
Zika transmission in South East Asia
The information on current Zika transmission is useful to evaluate the risk for people who have recently returned from or are planning to travel to countries with active local transmission.
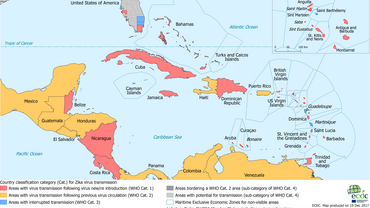
Zika transmission in the Caribbean
Zika transmission on december 2017.
This information aims at aiding diagnosis for returning travellers, especially pregnant women with travel history during pregnancy, returning from countries and territories that have recently experienced or are currently experiencing local Zika virus transmission .
Map and table
Zika transmission in past nine months.
Countries and territories worldwide with reported confirmed autochthonous vector-borne transmission of Zika virus infection in the past nine months. The information is provided to aid diagnosis for returning travellers, especially pregnant women
- Skip to main content
- Skip to "About this site"
Language selection
Search travel.gc.ca.
Help us to improve our website. Take our survey !
Zika virus: Advice for travellers
Level 2 - practise enhanced health precautions ( more details ).
Original publication date: January 28, 2014
Updated: August 31, 2023
- The Current Situation and Health Professionals sections have been updated.
Current Situation
Zika virus continues to be a concern in many parts of the world. Transmission can occur in most areas of the world where the mosquito Aedes aegypti, the principal vector, occurs. This means that there is the potential for transmission through much of the tropical and subtropical world and beyond.
The risk of transmission to travellers is considered low.
To find out if your destination is a country or area with risk of Zika virus, consult the Travel Advice and Advisories page , and select your destination. Information on diseases spread by insects, such as Zika virus, is found under the ‘Health’ tab.
Zika virus typically causes mild illness lasting only a few days. Many people who are infected have no symptoms and do not know that they have been infected. Only 1 in 4 people infected with Zika virus develop symptoms.
Symptoms of Zika virus infection often include:
- conjunctivitis (pink eye)
- joint and muscle pain
A Zika virus infection in a pregnant woman can pose significant risks to the unborn baby, even if the woman does not develop any symptoms. Zika virus can cause serious birth defects including microcephaly (an abnormally small head), brain abnormalities, vision and hearing loss, and more. When some of these birth defects are present together, the condition is called Congenital Zika Syndrome (CZS).
There have also been increased reports of a serious nervous system disorder in adults, called Guillain-Barré syndrome , in countries and areas with risk of Zika virus.
Zika virus is primarily spread through the bite of an infected mosquito. It can also spread by:
- A pregnant woman infected with Zika virus passing the virus to her unborn baby.
- A person infected with Zika virus passing the virus through sexual contact. This includes contact with semen, vaginal fluid, blood or other body fluids during vaginal, anal or oral sex without a condom. This may also include the sharing of sex toys.
- A person infected with Zika virus who donates cells, blood, tissue, sperm (semen) or organs.
Currently, there is no vaccine to prevent or medication to treat infection with Zika virus. Symptoms, when present, will typically resolve on their own within a few days. Treatment aims to relieve symptoms.
Recommendations
For pregnant women or those planning a pregnancy
Zika virus infection during pregnancy increases the risk for serious birth defects since women can pass the virus to their unborn babies.
Pregnant women and women planning a pregnancy should visit a health care professional at least 6 weeks before travelling to discuss the potential risks of travelling to a country or area with risk of Zika virus. Pregnant women may choose to avoid or postpone travel to these areas.
Zika virus can be sexually transmitted. Infected men with or without symptoms, can carry Zika virus in their semen for a prolonged period of time. Partners should be aware of the risk so they can make informed travel decisions and take appropriate precautions.
Pregnant women should always use condoms correctly or avoid sexual contact with anyone who has travelled to a country or area with risk of Zika virus for the duration of their pregnancy.
For all travellers to countries or areas with risk of Zika virus
Before your trip
- Consult a health care professional or visit a travel health clinic at least 6 weeks before you travel.
During your trip
- Use approved insect repellent and apply it properly
- Cover up by wearing light-coloured, loose clothing, long pants and tucked-in long-sleeved shirts with closed-toe shoes or boots and a hat.
- Sleep in indoor areas that are completely enclosed or well-screened.
- Use mosquito netting (bed net) when sleeping outdoors or staying in a building that is not completely enclosed and to cover playpens, cribs or strollers.
- Learn more about mosquito bite prevention for travellers .
- Always use condoms correctly or avoid sexual contact while in countries or areas with risk of Zika virus.
After your trip
- See a health care professional if you had or currently have symptoms of Zika virus infection .
- Tell your health care professional:
- where you have been living or travelling, and
- if you have had unprotected sexual contact with someone who could be infected with Zika virus.
- Always use condoms correctly or avoid sexual contact for 2 months after travel or after onset of illness due to Zika virus (whichever is longer).
- Before trying for a pregnancy, wait 2 months after travel or after onset of illness due to Zika virus (whichever is longer) , to reduce the risk of passing the virus to your unborn baby. If your male partner travelled with you, wait 3 months after travel or after onset of illness due to Zika virus (whichever is longer) , to reduce the risk of sexual transmission.
- If you have a pregnant partner, always use condoms correctly or avoid sexual contact for the duration of the pregnancy.
- Before trying for a pregnancy or donating semen, wait 3 months after travel or after onset of illness due to Zika virus (whichever is longer) , to reduce the risk of sexual transmission.
- In all other situations, always use condoms correctly or avoid sexual contact for 3 months after travel or after onset of illness due to Zika virus (whichever is longer).
Information for Health Care Professionals
- The Committee to Advise on Tropical Medicine and Travel (CATMAT) has developed a statement on Prevention and Treatment of Zika virus.
- Zika virus: For health professionals
Registration of Canadians Abroad
Sign up with the Registration of Canadians Abroad service to stay connected with the Government of Canada in case of an emergency abroad or an emergency at home.
- World Health Organization - Zika virus classification tables
- World Health Organization - Zika virus disease
- Government of Canada – Zika virus
- Safer condom use
- If you become sick or injured while travelling outside Canada or after your return
Language selection
- Français fr
Zika virus: Latest travel health advice
- Symptoms and treatment
- Pregnant or planning a pregnancy
- Zika and men
- Prevention and risks
- For health professionals
- Latest travel health advice
Zika virus, along with other mosquito-transmitted diseases, such as malaria, dengue and chikungunya, are transmitted by mosquitoes that live in warmer climates. To determine if the area you are travelling to is a country or area with risk of Zika virus or a country or area with a Zika virus outbreak:
- Consult the Travel Advice and Advisories page , and select your destination. Information on diseases spread by insects, such as Zika virus, is found under the 'Health' tab.
- Consult a health care professional or visit a travel health clinic at least 6 weeks before you travel.
Canada posts travel-related information on travel.gc.ca.
Latest travel health notice on Zika:
Advice for travellers: Zika Travel Health Notice
Page details
You are using an outdated browser. Upgrade your browser today or install Google Chrome Frame to better experience this site.
Travel Health Notices
Search By Country Name or Disease
CDC uses Travel Health Notices (THNs) to inform travelers about global health risks during outbreaks, special events or gatherings, and natural disasters, and to provide advice about protective actions travelers can take to prevent infection or adverse health effects.
A THN can be posted for: 1) a disease outbreak (higher number of expected cases) in a country or region, 2) sporadic cases of a disease in an unusual or new geographic location, 3) natural and human-made disasters with severe environmental health risks, or infrastructure damage that would limit healthcare services availability and 4) mass gathering events that can lead to disease outbreaks. See types of travel notices .
Level 4 - Avoid All Travel
- Currently there are no Travel Health Notices at this level.
Level 3 - Reconsider Nonessential Travel
Level 2 - practice enhanced precautions.
- There is an outbreak of chikungunya in the Malé and Hulhumalé regions of Maldives. Mosquitoes spread the virus that causes chikungunya.Chikungunya in Maldives761 New Chikungunya in Maldives May 28, 2024 There is an outbreak of chikungunya in the Malé and Hulhumalé regions of Maldives. Mosquitoes spread the virus that causes chikungunya. Read More >>
- Some international destinations have circulating poliovirus. Before any international travel, make sure you are up to date on your polio vaccines. Country List : Afghanistan, Algeria, Benin, Cameroon, Central African Republic, Chad, Côte d'Ivoire (Ivory Coast), Democratic Republic of the Congo, Madagascar, Malawi, Mozambique, Niger, Nigeria, Pakistan, Somalia, Yemen, Indonesia, Sudan, Mali, Botswana, Zambia, Republic of the Congo , Burundi, Burkina Faso, Kenya, Tanzania, including Zanzibar, Guinea, Mauritania, Egypt, Zimbabwe, Angola, Liberia, Senegal, Sierra LeoneGlobal Polio734 Global Polio May 23, 2024 Some international destinations have circulating poliovirus. Before any international travel, make sure you are up to date on your polio vaccines. Destination List: Afghanistan, Algeria, Angola, Benin, Botswana, Burkina Faso, Burundi, Cameroon, Central African Republic, Chad, Côte d'Ivoire (Ivory Coast), Democratic Republic of the Congo, Egypt, Guinea, Indonesia, Kenya, Liberia, Madagascar, Malawi, Mali, Mauritania, Mozambique, Niger, Nigeria, Pakistan, Republic of the Congo, Senegal, Sierra Leone, Somalia, Sudan, Tanzania, including Zanzibar, Yemen, Zambia, Zimbabwe Read More >>
- There are confirmed and suspect cases of diphtheria in several regions in Guinea. Vaccination against diphtheria is essential to protect against disease. If you are traveling to an affected area, you should be up to date with your diphtheria vaccines.Diphtheria in Guinea751 Diphtheria in Guinea April 23, 2024 There are confirmed and suspect cases of diphtheria in several regions in Guinea. Vaccination against diphtheria is essential to protect against disease. If you are traveling to an affected area, you should be up to date with your diphtheria vaccines. Read More >>
- There is an outbreak of chikungunya in Timor-Leste.Chikungunya in Timor-Leste759 Chikungunya in Timor-Leste April 05, 2024 There is an outbreak of chikungunya in Timor-Leste. Read More >>
- Yellow fever cases remain elevated in Nigeria, after an outbreak was first reported in November 2020. Travelers to Nigeria should take steps to prevent yellow fever by getting vaccinated at least 10 days before travel and taking steps to prevent mosquito bites.Yellow Fever in Nigeria392 Yellow Fever in Nigeria March 28, 2024 Yellow fever cases remain elevated in Nigeria, after an outbreak was first reported in November 2020. Travelers to Nigeria should take steps to prevent yellow fever by getting vaccinated at least 10 days before travel and taking steps to prevent mosquito bites. Read More >>
- There is an outbreak of diphtheria in Niger. If you are traveling to an affected area, you should be up to date with your diphtheria vaccines.Diphtheria in Niger752 Diphtheria in Niger February 25, 2024 There is an outbreak of diphtheria in Niger. If you are traveling to an affected area, you should be up to date with your diphtheria vaccines. Read More >>
- There is an outbreak of diphtheria in several states in Nigeria. Vaccination against diphtheria is essential to protect against disease. If you are traveling to an affected area, you should be up to date with your diphtheria vaccines.Diphtheria in Nigeria 740 Diphtheria in Nigeria February 16, 2024 There is an outbreak of diphtheria in several states in Nigeria. Vaccination against diphtheria is essential to protect against disease. If you are traveling to an affected area, you should be up to date with your diphtheria vaccines. Read More >>
- There is an outbreak of mpox in 22 out of 26 provinces, including urban areas, in the DRC.Mpox in the Democratic Republic of the Congo438 Mpox in the Democratic Republic of the Congo February 16, 2024 There is an outbreak of mpox in 22 out of 26 provinces, including urban areas, in the DRC. Read More >>
Level 1 - Practice Usual Precautions
- There are outbreaks of Oropouche fever in parts of Brazil, Bolivia, Peru, Colombia, and Cuba. Travelers to affected areas should take steps to avoid bug bites. Country List : Brazil, Bolivia, Peru, Colombia, CubaOropouche Fever in the Americas758 Oropouche Fever in the Americas June 05, 2024 There are outbreaks of Oropouche fever in parts of Brazil, Bolivia, Peru, Colombia, and Cuba. Travelers to affected areas should take steps to avoid bug bites. Destination List: Bolivia, Brazil, Colombia, Cuba, Peru Read More >>
- Many international destinations are reporting increased numbers of cases of measles. Country List : Afghanistan, Angola, Benin, Cameroon, Central African Republic, Chad, Côte d'Ivoire (Ivory Coast), Democratic Republic of the Congo, Djibouti, Ethiopia, Gabon, India, Indonesia, Liberia, Niger, Nigeria, Pakistan, Republic of the Congo , Senegal, Somalia, Republic of South Sudan, Sudan, Tajikistan, Togo, Yemen, Zambia, Nepal, Kyrgyzstan, Armenia, Mauritania, Lebanon, Equatorial Guinea, Syria, Ghana, Kazakhstan, Burkina Faso, Turkey, Qatar, United Arab Emirates, Libya, Burundi, Romania, Malaysia, Russia, Azerbaijan, Sri Lanka, Uzbekistan, Philippines, Austria, BelarusGlobal Measles743 Updated Global Measles May 28, 2024 Many international destinations are reporting increased numbers of cases of measles. Destination List: Afghanistan, Angola, Armenia, Austria, Azerbaijan, Belarus, Benin, Burkina Faso, Burundi, Cameroon, Central African Republic, Chad, Côte d'Ivoire (Ivory Coast), Democratic Republic of the Congo, Djibouti, Equatorial Guinea, Ethiopia, Gabon, Ghana, India, Indonesia, Kazakhstan, Kyrgyzstan, Lebanon, Liberia, Libya, Malaysia, Mauritania, Nepal, Niger, Nigeria, Pakistan, Philippines, Qatar, Republic of South Sudan, Republic of the Congo, Romania, Russia, Senegal, Somalia, Sri Lanka, Sudan, Syria, Tajikistan, Togo, Turkey, United Arab Emirates, Uzbekistan, Yemen, Zambia Read More >>
- The Hajj, or pilgrimage to Mecca, Saudi Arabia, is one of the world’s largest mass gatherings. Mass gatherings, such as Hajj or Umrah, can increase the risk for infections such as meningococcal disease.Meningococcal Disease in Saudi Arabia - Vaccine Requirements for Travel During the Hajj and Umrah Pilgrimages146 New Meningococcal Disease in Saudi Arabia - Vaccine Requirements for Travel During the Hajj and Umrah Pilgrimages May 20, 2024 The Hajj, or pilgrimage to Mecca, Saudi Arabia, is one of the world’s largest mass gatherings. Mass gatherings, such as Hajj or Umrah, can increase the risk for infections such as meningococcal disease. Read More >>
- Dengue is a risk in many parts of Central and South America, Mexico, and the Caribbean. Some countries are reporting increased numbers of cases of the disease. Travelers to the Americas can protect themselves by preventing mosquito bites. Country List : Colombia, Guatemala, Nicaragua, Panama, Guadeloupe, Martinique (France), Costa Rica, French Guiana (France), Mexico, Brazil, Paraguay, Argentina, Peru, Ecuador, including the Galápagos Islands, Uruguay, Curaçao, Guyana, HondurasDengue in the Americas427 Dengue in the Americas May 16, 2024 Dengue is a risk in many parts of Central and South America, Mexico, and the Caribbean. Some countries are reporting increased numbers of cases of the disease. Travelers to the Americas can protect themselves by preventing mosquito bites. Destination List: Argentina, Brazil, Colombia, Costa Rica, Curaçao, Ecuador, including the Galápagos Islands, French Guiana (France), Guadeloupe, Guatemala, Guyana, Honduras, Martinique (France), Mexico, Nicaragua, Panama, Paraguay, Peru, Uruguay Read More >>
- Dengue is a risk in many parts of Africa and the Middle East. Some countries are reporting increased numbers of cases of the disease. Travelers to Africa and the Middle East can protect themselves by preventing mosquito bites. Country List : Sudan, Burkina Faso, Mali, Ethiopia, MauritiusDengue in Africa and the Middle East428 Dengue in Africa and the Middle East May 16, 2024 Dengue is a risk in many parts of Africa and the Middle East. Some countries are reporting increased numbers of cases of the disease. Travelers to Africa and the Middle East can protect themselves by preventing mosquito bites. Destination List: Burkina Faso, Ethiopia, Mali, Mauritius, Sudan Read More >>
- Dengue is a risk in many parts of Asia and the Pacific Islands. Some countries are reporting increased numbers of cases of the disease. Travelers to Asia and the Pacific Islands can protect themselves by preventing mosquito bites. Country List : Sri Lanka, Cambodia, Indonesia, Laos, Singapore, Fiji, SamoaDengue in Asia and the Pacific Islands429 Dengue in Asia and the Pacific Islands May 16, 2024 Dengue is a risk in many parts of Asia and the Pacific Islands. Some countries are reporting increased numbers of cases of the disease. Travelers to Asia and the Pacific Islands can protect themselves by preventing mosquito bites. Destination List: Cambodia, Fiji, Indonesia, Laos, Samoa, Singapore, Sri Lanka Read More >>
- There are reports of Western equine encephalitis virus (WEEV) infections in horses, humans or both, in parts of Argentina and Uruguay. Country List : Argentina, UruguayWestern Equine Encephalitis Virus in South America760 Western Equine Encephalitis Virus in South America March 14, 2024 There are reports of Western equine encephalitis virus (WEEV) infections in horses, humans or both, in parts of Argentina and Uruguay. Destination List: Argentina, Uruguay Read More >>
- There have been reports of Rocky Mountain spotted fever (RMSF) in people traveling to the United States from Tecate, in the state of Baja California, Mexico.Rocky Mountain Spotted Fever in Mexico 756 Rocky Mountain Spotted Fever in Mexico March 12, 2024 There have been reports of Rocky Mountain spotted fever (RMSF) in people traveling to the United States from Tecate, in the state of Baja California, Mexico. Read More >>
- An outbreak of extensively drug-resistant (XDR) typhoid fever in Pakistan is ongoing. Extensively drug-resistant infections do not respond to most antibiotics.XDR Typhoid Fever in Pakistan397 XDR Typhoid Fever in Pakistan June 16, 2023 An outbreak of extensively drug-resistant (XDR) typhoid fever in Pakistan is ongoing. Extensively drug-resistant infections do not respond to most antibiotics. Read More >>
- Some travelers who have spent time in Mexico have been infected with multidrug-resistant (MDR) Salmonella Newport. Salmonella Newport in Mexico732 Salmonella Newport in Mexico March 29, 2023 Some travelers who have spent time in Mexico have been infected with multidrug-resistant (MDR) Salmonella Newport. Read More >>
Types of Notices
Visit U.S. Department of State's website for the latest Travel Advisories .
File Formats Help:
- Adobe PDF file
- Microsoft PowerPoint file
- Microsoft Word file
- Microsoft Excel file
- Audio/Video file
- Apple Quicktime file
- RealPlayer file
- Zip Archive file
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.

IMAGES
VIDEO
COMMENTS
Zika is spread mostly by the bite of an infected Aedes species mosquito ( Ae. aegypti and Ae. albopictus ). Zika can be passed from a pregnant woman to her fetus. Infection during pregnancy can cause certain birth defects. Your decision to delay or cancel travel is personal and complex.
Treatment and Prevention of Zika Virus Disease. Review information for healthcare providers on how to treat and prevent Zika virus disease. Sexual Transmission of Zika Virus. Clinical Testing and Diagnosis for Zika Virus Disease. Clinical Considerations for Pregnant Persons with Possible Zika Virus Infection.
Zika in the US; Zika Travel Information; Guillain-Barré Syndrome. ... The webpage includes a world map of areas with risk of Zika for other countries and territories outside of U.S. states. A new ...
Zika virus is primarily transmitted by the bite of an infected mosquito from the Aedes genus, mainly Aedes aegypti , in tropical and subtropical regions. Aedes mosquitoes usually bite during the day, peaking during early morning and late afternoon/evening. This is the same mosquito that transmits dengue, chikungunya and yellow fever.
Overview . In light of ongoing transmission and sporadic clusters of infection in multiple regions where Aedes (Stegomyia) mosquitoes are present, World Health Organization has compiled information for travelers to be aware of the risk and routes of transmission, potential for complications of Zika virus infection during pregnancy, and how to take steps to avoid becoming infected.
Zika transmission. Zika transmission maps have stopped being updated in January 2018, as a result as a result of the significant slow-down of the epidemic. ECDC is working with the World Health Organization and the United States CDC to assess the current risk of Zika virus transmission in countries and territories globally. Information on the ...
Travel to areas with Zika could include: Zika is a serious concern for a pregnant woman's developing baby, and infection during pregnancy can cause severe birth defects. Women and their sexual partners who must go to areas with risk of Zika should prevent mosquito bites and avoid having unprotected sex during and after travel. Couples planning ...
Zika virus can also be transmitted through sex and during pregnancy from mother to foetus. Aedes mosquitoes also transmit dengue, chikungunya, yellow fever, and other viruses. These mosquito-transmitted viruses often circulate in the same geographic areas. Based on available evidence, WHO advises against any restriction of travel to or trade ...
Zika is a risk in multiple countries and territories. The best way to protect yourself from Zika is to avoid travel to areas with risk of the virus. Use the CDC's map to find out where it is safe to travel.
Countries and territories with current or previous Zika virus transmission MAP DATE: 17 October 2018 The boundaries and names shown and the designations used on this map do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of
• Zika can stay in semen for months and can be passed through sex. • If a woman gets pregnant and is infected with Zika, it can cause serious birth defects. If you and your partner are thinking about pregnancy: • Talk to a doctor before you travel to an area with risk of Zika. • If you do travel, wait to get pregnant.
For all travellers to countries or areas with risk of Zika virus. Before your trip. Consult a health care professional or visit a travel health clinic at least 6 weeks before you travel. During your trip. Prevent mosquito bites at all times. The mosquitoes that spread Zika virus bite during the day and night.
Latest travel health advice. Zika virus, along with other mosquito-transmitted diseases, such as malaria, dengue and chikungunya, are transmitted by mosquitoes that live in warmer climates. To determine if the area you are travelling to is a country or area with risk of Zika virus or a country or area with a Zika virus outbreak:
CDC uses Travel Health Notices (THNs) to inform travelers about global health risks during outbreaks, special events or gatherings, and natural disasters, and to provide advice about protective actions travelers can take to prevent infection or adverse health effects. A THN can be posted for: 1) a disease outbreak (higher number of expected ...