This website requires cookies. Please accept or refuse these cookies first. For more info about our cookies, please read our cookies policy .

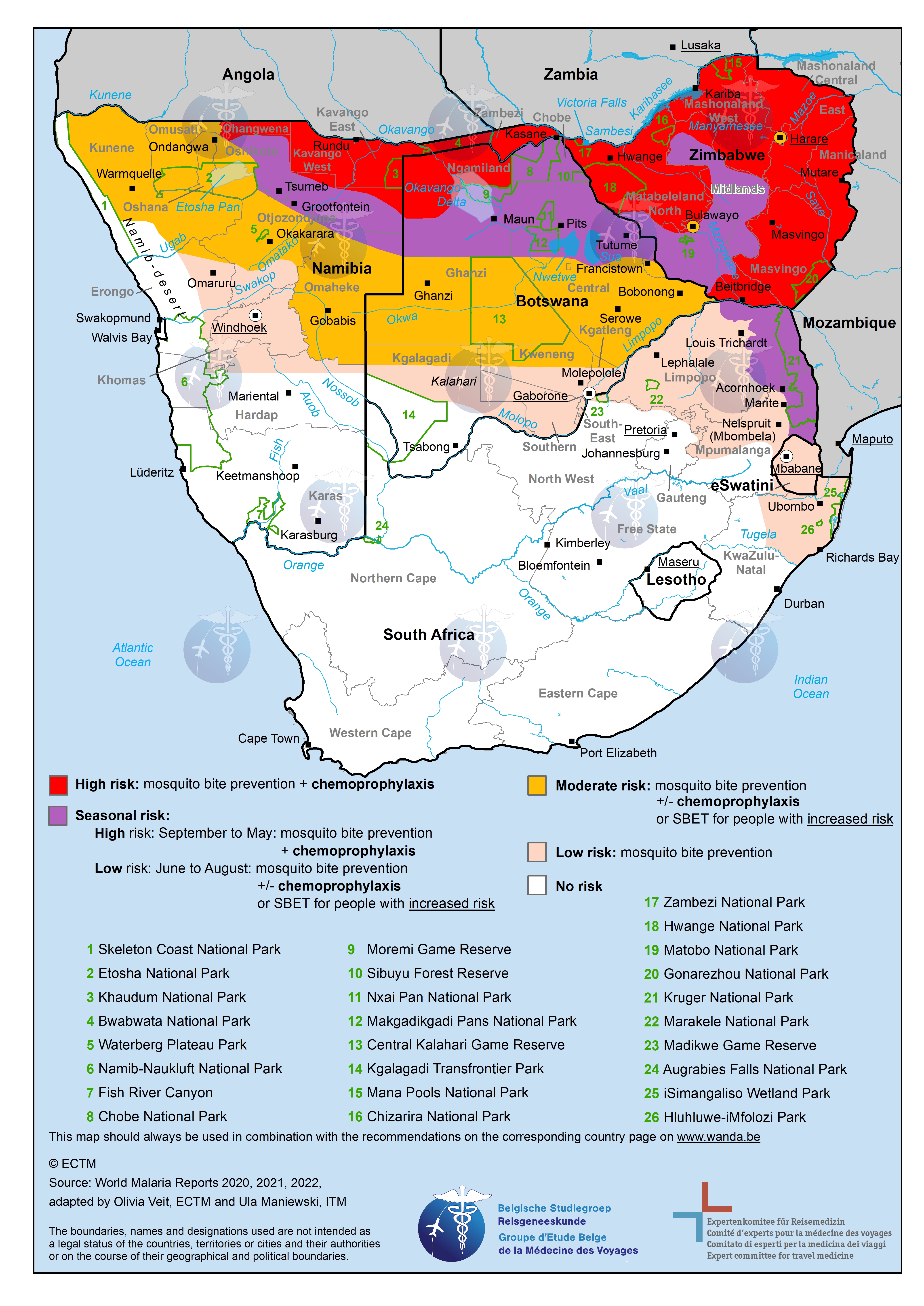
Malaria - map of South Africa
Map of south africa with malaria risk areas.
This image shows a map with the countries of South Africa. Regions with a low risk of malaria are marked in light pink, regions with a moderate risk are marked in orange, regions with a high risk are marked in red and regions with a seasonal risk are marked in purple. This information, and the recommended preventive measures, can be looked up in detail on the separate pages for each country .
View a larger version of the map by clicking on it.
More information about malaria .

Mapping malaria in Africa: climate change study predicts where mosquitoes will breed in future
Professor of Water Science & Heath, University of Leeds
Global Professor in Water & Planetary Health, University of Lincoln
Disclosure statement
Mark Smith receives funding from the UK Natural Environment Research Council.
Chris Thomas receives funding from the UK Natural Environment Research Council.
University of Leeds provides funding as a founding partner of The Conversation UK.
University of Lincoln provides funding as a member of The Conversation UK.
View all partners
The relationship between climate and malaria transmission is complex and has been the subject of intense study for some three decades.
Mosquito vector populations sufficient to maintain malaria transmission occur within a particular range of temperatures and humidity that are suitable for their survival and breeding. The parasite also needs suitable temperatures to complete its mosquito life stages. And mosquitoes need surface water to breed in. These conditions have to last long enough for mosquito and parasite populations to grow.
Much of sub-Saharan Africa provides exactly these conditions. Factors like public health interventions, land use, urbanisation and quality of housing also determine transmission and local disease burden. But a suitable climate is a big factor in explaining the latest available data (from 2022). This shows that 94% of the 249 million global malaria cases are recorded in Africa and that nearly all of the 608,000 global malaria deaths annually are on the continent.
Climate change is likely to cause a shift in the suitability for transmission in some areas.
It’s fairly straightforward to model the effect of changing temperatures on malaria by using climate data and the thermal ranges of vector and parasite. Rainfall data is less useful, because mosquitoes breed in shallow, slow-moving or standing water, often in very small water bodies such as puddles. And rain doesn’t normally stay where it lands. This is where hydrology – the study of water’s movement and how it’s distributed – becomes useful in modelling.
We are part of an interdisciplinary team that has just published a new set of estimates for future malaria suitability across the African continent in the journal Science. Our work incorporates the dynamics of water flows and stores that can influence breeding locations. The results give a more accurate picture than before of where the malaria transmission season might get longer or shorter as the climate changes.
We found that overall malaria suitability will decrease, especially in west Africa. But other areas, particularly river corridors and floodplains, will become more suitable for malaria transmission. Massive population growth is expected across Africa over the next 25 years, often located close to rivers. This means the number of people living in potentially malaria endemic areas (suitable for transmission more than nine months a year) will increase by 2100 to over a billion.
Along with knowledge of the breeding habitat preferences of specific mosquitoes and their human biting preferences (indoor or outdoor, dusk or night time), this information could help to target and tailor malaria control plans.
Follow the water
Building on our earlier pilot study , published in 2020, in this new study we used seven global hydrological models. Each was run using four climate models. We looked at different possible futures by including a low-, medium- and high-emissions scenario for greenhouse gases.
Read more: Malaria: new map shows which areas will be at risk because of global warming
Thanks to this approach we can now include many hydrological processes such as the soaking of water into the earth, evaporation of water back into the atmosphere and the flow of water through the landscape in large rivers.
This offers the most sophisticated representation yet of potential malaria vector breeding sites across Africa and how these might change in future.
A new picture emerges
Our models paint a complex and realistic pattern of malaria transmission suitability today and in future. Unlike previous work, our model highlights waterways and floodplains as potentially suitable mosquito breeding sites, often on their margins or isolated water bodies nearby.
For instance, the Nile corridor in Egypt is omitted in previous thermal and rainfall models. But when hydrology is included, as in our study, the area is predicted to be highly suitable for malaria transmission.
Egypt is currently malaria-free due to extensive control efforts . It nonetheless remains climatically suitable and malaria mosquitoes can still be found there. We know that malaria was present there until the 1990s and traces of the malaria parasite have even been found in ancient Egyptian mummies .
Read more: From malaria, to smallpox, to polio – here's how we know life in ancient Egypt was ravaged by disease
Changing suitability
Overall, we found that by 2100, a general decrease in malaria suitability is projected across the majority of Africa. Future climates are increasingly either too warm or too dry for year-round malaria transmission.
The main location of this decrease is centred on west Africa around The Gambia. It extends right across the continent at that latitude as far as South Sudan. We also see smaller decreases in suitability in southern Africa around Botswana and Zimbabwe.
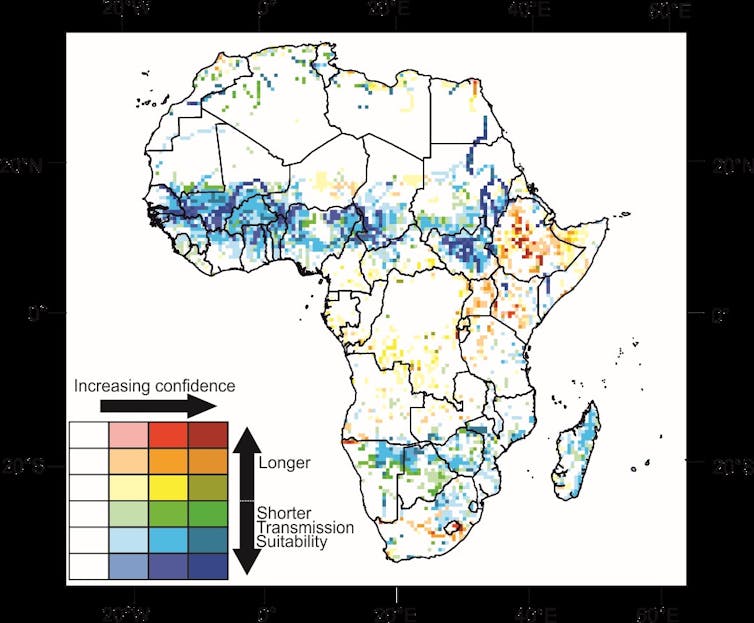
This reduction in transmission season length is also seen in previous studies using rainfall to represent surface water. But using hydrological models reveals more concentrated and bigger reductions in season length. The reduced suitability was most pronounced in the high-emissions scenario for greenhouse gases.
Crucially, though, the outcome in our hydrology-driven model was particularly sensitive to future greenhouse gas emissions.
Some areas, particularly around the highlands of Ethiopia, are seen to increase in suitability by 2100, driven by increases in temperature in the cooler mountains. We can also see an increase in malaria suitability following the course of the Orange River in South Africa, where local malaria plans are focused on avoiding re-introduction of transmission along the river.
Is this good news?
Reducing malaria suitability in Africa is a good thing. But when the climate is either too warm or too dry for the malaria parasite or mosquito to survive, there will be other adverse outcomes, particularly for water supply and agriculture. By including water flows in malaria suitability estimates, we can start to examine interactions with these other sectors more directly.
- Climate change
- Climate models
- Malaria transmission
- Climate crisis
- Malaria vector
- Greenhouse gas emissions (GHG)
Want to write?
Write an article and join a growing community of more than 184,200 academics and researchers from 4,969 institutions.
Register now
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Springer Nature - PMC COVID-19 Collection

Exploring public awareness of the current and future malaria risk zones in South Africa under climate change: a pilot study
Jennifer m fitchett.
School of Geography, Archaeology and Environmental Studies, University of the Witwatersrand, Johannesburg, South Africa
Deryn-Anne Swatton
Although only a small proportion of the landmass of South Africa is classified as high risk for malaria, the country experiences on-going challenges relating to malaria outbreaks. Climate change poses a growing threat to this already dire situation. While considerable effort has been placed in public health campaigns in the highest-risk regions, and national malaria maps are updated to account for changing climate, malaria cases have increased. This pilot study considers the sub-population of South Africans who reside outside of the malaria area, yet have the means to travel into this high-risk region for vacation. Through the lens of the governmental “ABC of malaria prevention”, we explore this sub-population’s awareness of the current boundaries to the malaria area, perceptions of the future boundary under climate change, and their risk-taking behaviours relating to malaria transmission. Findings reveal that although respondents self-report a high level of awareness regarding malaria, and their boundary maps reveal the broad pattern of risk distribution, their specifics on details are lacking. This includes over-estimating both the current and future boundaries, beyond the realms of climate-topographic possibility. Despite over-estimating the region of malaria risk, the respondents reveal an alarming lack of caution when travelling to malaria areas. Despite being indicated for high-risk malaria areas, the majority of respondents did not use chemoprophylaxis, and many relied on far less-effective measures. This may in part be due to respondents relying on information from friends and family, rather than medical or governmental advice.
Introduction
Malaria is a dangerous and potentially fatal vector-borne disease caused by the Plasmodium parasite, transmitted to humans through the bite of an infected female Anopheles mosquito (Cohuet et al. 2010 ; Cella et al. 2019 ). In 2018, an estimated 228 million cases of malaria were reported globally, similar to 231 million cases for 2017, with a death toll of ~ 435,000 in 2017 and 405,000 in 2018 (Alonso and Noor 2017 ; WHO 2019a , b ). The burden of malaria was disproportionately borne by developing nations, particularly in the African continent, which accounted for 92% and 93% of the malaria cases and deaths recorded in 2018, respectively (Alonso and Noor 2017 ; WHO 2019b ). In 2016, in an effort to combat malaria, the World Health Organization launched the E-2020 initiative in which it identified 21 countries with the potential to eliminate malaria within their borders by the year 2020 (WHO 2018 ). South Africa was one of those countries, with an additional local objective of elimination by 2018 (Baker 2018 ). However, in 2017, South Africa experienced a severe setback in achieving both objectives, reporting 7897 imported and 19,706 indigenous cases of malaria—the highest number of cases reported by any of the 21 identified countries, and more than four times the number of indigenous cases reported in the country in 2016 (WHO 2018 ; Maharaj et al. 2019 ; Abiodun et al. 2020 ). This increase in numbers was due to a range of factors, including an increase in rainfall, temperature, and humidity following an abnormally mild winter (Baker 2018 ). As a result, the World Health Organization has classed South Africa as “off-track” and has made a number of recommendations as to how the country can steer itself back on course (WHO 2018 ). Among these are efforts to refine malaria risk maps, to improve public awareness, and to revisit malaria strategies (WHO 2018 ). Due to the unlikelihood of achieving malaria elimination within South Africa by 2020, the South African government has now adopted the self-mandated goal of zero malaria transmission by 2023, which it announced in the Malaria Elimination Strategic Plan for South Africa 2019–2023 (National Department of Health 2019 ). One of the primary objectives of the Malaria Elimination Strategic Plan is to “ensure that 90% of the population affected by malaria receives information and education communication messaging by 2023” (National Department of Health 2019 : 25). This objective speaks directly to the recommendations given by the World Health Organization (WHO 2018 ). The National Guidelines for the Prevention of Malaria in South Africa comprise five key components which are summarized as the “ABC” of malaria prevention, namely Awareness and Assessment of malaria risk, avoidance of mosquito Bites, Compliance with Chemoprophylaxis when indicated, early Detection of malaria disease, and Effective treatment (NDOH 2018a ; Baker 2018 ; Schmidt 2019a , b ).
Adding fuel to the fire, climate change is resulting in both an increase in the extent of the South African malaria risk area and the incidence of malaria transmission due to increases in temperature and changes in rainfall patterns (Morris et al., 2013 ; Abiodun et al., 2020 ). The spatial distribution of malaria globally is strongly determined by climate (Caminade et al. 2014 ; Cella et al. 2019 ). By 2050, a 50% increase in the probability of malaria incidence is projected globally due to increases in temperature and changes in rainfall patterns (Cella et al. 2019 ). Temperature has been found to affect both the extent of malaria areas and malaria transmission incidence across the African continent (Eikenberry and Gumel 2019 ). For South Africa, both temperature and rainfall have been found to affect the malaria rate, with a notable increase in cases following flood events induced by strong tropical storms (Adeola et al. 2019 ; Makinde and Abiodun 2019 ). A change in malaria area extent is documented in the frequent updating of the National Department of Health malaria risk maps for South Africa (Coetzee et al. 2013 ; Morris et al. 2013 ). Mathematical models have been developed to explore the role of climate in malaria incidence and to aid in projecting future changes in malaria risk distribution, and the risk of transmission (Cella et al. 2019 ; Eikenberry and Gumel 2019 ). At a local scale, seasonal malaria forecast models are being developed for South Africa (Kim et al. 2019 ; Landman et al. 2020 ). The modelling of the climate impact on malaria distribution and incidence and projections for future incidence are valuable in developing public health policies, and in communication to the public. However, the increased incidence of malaria cases in South Africa in recent years would suggest that effectiveness of the distribution of these maps, particularly with regular updates, remains limited.
A range of studies relating to disparate diseases have revealed the importance of public awareness and understanding of disease risk in determining the precautionary measures that they will adopt (cf. Erhardt and Hobbs 2002 ; Glik et al. 2004 ; Goldman et al. 2006 ; Young et al. 2008 Seale et al. 2010 ). The role of sources of information, and the level of frequency of communication regarding diseases, is key in the veracity of public awareness (Young et al. 2008 ). This has been widely understood in South African malaria control efforts (Blumberg et al. 2014 ), with malaria risk maps for the public dating back to 1938 (Coetzee et al. 2013 ). However, the efforts in assessing communities’ awareness and understanding of malaria risk have largely been limited to regions within the high-risk zone, due to resource scarcity (Maartens et al. 2007 ; Cox et al. 2018 ).
In line with the WHO ( 2018 ) recommendations, this pilot study seeks to expand on previous work to explore public understanding of the location of the contemporary high-risk malaria zone, the anticipated future expansion of the high-risk malaria zone under climate change, and public behaviour regarding malaria precaution and prophylaxis, among those who reside outside of the malaria risk area, but travel into the high-risk zone on vacation. The aim of this study is to develop a methodology to explore any disconnects between reported and demonstrated awareness and behaviour regarding malaria for gaps in understanding to be addressed more effectively through government intervention, and for targeted awareness campaigns to be developed.
Malaria can be found in each of South Africa’s neighbouring countries, barring Lesotho, where the altitude and consequent colder temperatures make it inhospitable to the malaria-carrying Anopheles mosquito (Blumberg et al. 2014 ). Within South Africa itself, malaria is confined to the north-eastern reaches of the country, where it is endemic to three of South Africa’s nine provinces, namely Limpopo, Mpumalanga, and northern KwaZulu-Natal (Maharaj et al. 2019 ; Adeola et al. 2019 ; Fig. 1 ). According to the latest malaria risk map produced by the South African National Department of Health in December 2018, areas of moderate risk (where chemoprophylaxis is indicated for all travellers from September to May) are concentrated along the border between South Africa and Mozambique and Zimbabwe (Fig. (Fig.1). 1 ). These areas of moderate risk can be found in the Mpumalanga municipality district of Ehlanzeni and in Limpopo in the Mopani and Vhembe municipality districts (Fig. (Fig.1). 1 ). Areas of low risk (where malaria is still present, but only non-drug preventative measures are indicated), however, extend as far west as Limpopo’s Waterberg district and as far south as the Umkhanyakude district in KwaZulu-Natal Province (Fig. (Fig.1). 1 ). At present, the regions of highest malaria risk in South Africa have relatively low population density, with no major cities and a large proportion of the high-risk area limited to the Kruger National Park. This does however pose a threat to both local and international tourists visiting the Kruger National Park and nearby game farms and nature reserves, who may be unaware of the risk levels within South Africa.

South African malaria risk map produced by the National Department of Health in December 2018 (After NDoH 2018b )
This research explores respondents’ malaria awareness and self-reported behaviours. Therefore, questionnaires were selected as the most appropriate instrument for data collection. Types of questions ranged from multiple choice and Likert scale questions to more open-ended, paragraph-style questions (Bird, 2009 ). Topics included respondents’ demographics and travel histories, awareness and perception of malaria distribution and risk in Southern Africa, understanding of climate change, and attitudes towards malaria prophylaxis—both in terms of bite prevention and chemoprophylaxis. The closing question of the questionnaire required respondents to draw two lines, in different colours, on a map of Southern Africa: a blue line indicating where they understand the boundary of the present malaria risk zone to be located and a red line indicating where they think the boundary of the malaria risk zone will be located in 20 years’ time. The map was created using ArcMap and displayed national and provincial borders, but contained no other information to avoid leading respondents. Questionnaires were distributed in hard copy.
Respondents were identified through a combination of purposive and snowball sampling (Bernard 2013 ). To ensure a degree of standardization throughout the study sample for this initial pilot study, a target population was identified and a number of parameters were defined. In order to qualify for the study, respondents had to live within the Gauteng Province of South Africa (currently not a malaria area), have at least an undergraduate university degree, and have travelled to a malaria risk area within southern Africa within the last 5 years for leisure purposes. This ensured that the data gathered were relevant and that all respondents were middle- to upper-class leisure tourists with financial means to access to a wide range of precautionary measures to avoid contracting malaria when travelling to malaria areas, and have a reasonable level of education and understanding of health risks. This sample is not intended to be representative of the population of South Africa nor the Gauteng Province. Rather, this subgroup is used to determine whether any issues in awareness, behaviour, or use of chemoprophylaxis may warrant further, more extensive investigation. Ethics clearance was obtained from the University of the Witwatersrand’s Human Research Ethics Committee (non-medical) prior to entering the field (ethics clearance: gaes-2019-06-01). Given the target population and the minimum requirement of a completed undergraduate degree, no individuals under the age of 18 were approached. Finally, it is important to note that this research dealt exclusively with the data collected from the completed questionnaires and no medical records were accessed or used in this study.
Responses to closed-ended questions from the questionnaire were analyzed using descriptive statistics, while responses to open-ended questions underwent thematic coding and content analysis (Bernard 2013 ). The hand-drawn maps generated in the last question of the questionnaire were digitized and layered over one another to create composite maps showing areas of consensus/disagreement among respondents. This particular methodology was adapted from a study conducted by Roffe et al. ( 2019 ) which mapped agreement among experts regarding rainfall seasonality in South Africa. Finally, the composite maps were compared to the official malaria risk map created by the South African National Department of Health in 2018 to assess their accuracy.
A total of 38 questionnaires were completed. Of these, six questionnaires were excluded from analysis as the respondents did not fall within the defined parameters of the target population, in most instances as a result of not having visited a malaria area within the past 5 years. Four maps were deemed unusable as the lines drawn could not be digitized as a contiguous vector line and/or what was drawn could not be meaningfully interpreted. Neither the excluded questionnaires nor the unusable maps were used in the creation of the composite maps. In the cases where maps were unusable, but respondents met the selection criteria, the questionnaire responses were captured and included in descriptive statistics and content analysis.
While the intention was to obtain a relatively even distribution of respondents across age groups, due to difficulty accessing people within the older age brackets who met the selection criteria (specifically the criterion relating to tertiary education), a skewed age distribution resulted. Of the 32 respondents who submitted admissible questionnaires, the highest number ( n = 14) fell into the 20–29 age group. The remaining age groups were comparatively poorly represented, with the 30–39 and 40–49 age categories comprising five respondents each, and the older age groups (50–59 and 60+) each representing one-eighth ( n = 4) of the sample group. The majority ( n = 17) of respondents held only a Bachelor’s degree, while nine held Honours degrees (a postgraduate qualification in South Africa), one held a Master’s degree, and two held a PhD. The remaining three respondents selected “Other” qualifications, in two cases citing university qualifications in the medical or nursing field. This last group, however, cannot be read independently, as respondents with medical or similar degrees may equally have chosen the equivalent qualification to their degree-type from the list provided. The overall spread of tertiary qualifications indicates a well-educated subset of the population. It is worth noting that the various levels of education were spread across the age groups, and it was not simply a case of the older the respondent, the higher their qualification. For example, all respondents in the 40 to 49-year-old age group ( n = 5) reported only holding a Bachelor’s degree, whereas of the two respondents in possession of a PhD, one fell into the 30–39 age group and the other into the 60+ age group. This distribution is important, as it means that age and highest qualification are not collinear, but are rather independent factors each of which could be possible determinants of perception, risk assessment, and behaviour. We reiterate that the results of this small group cannot be read as representative of any sub-population, but rather the variety of results indicates a heterogeneity which reveals the importance of further, extensive, investigation.
A – A wareness and A ssessment of malaria risk
The questionnaire included a mapping exercise in which respondents were required to draw a line across a map of Southern Africa indicating where they understood the boundary of the present malaria risk zone to be located. In this question, respondents were instructed to include the entirety of the Southern African region in the drawing of their malaria zone boundaries. However, eight of the 28 respondents who created usable maps only drew lines across South Africa. Therefore, for the sake of consistency, only the portion of the drawn risk areas which fell within the borders of South Africa was digitized.
Overall, the composite map created from all 28 usable maps reveals a fairly high degree of awareness among respondents regarding the general location of malaria risk areas in South Africa (Fig. 2 ). Darker blue areas, which show a higher degree of consensus among respondents, can be found in the north-east of the country, concentrated along the border between South Africa and Mozambique and Zimbabwe (Fig. (Fig.2). 2 ). The majority of respondents have also included ESwatini, northern KwaZulu-Natal, and more westerly parts of Limpopo and Mpumalanga in their assessment of malaria risk areas (Fig. (Fig.2). 2 ). This is consistent with the official South African malaria risk map produced by the National Department of Health in 2018 (Fig. (Fig.1). 1 ). However, the composite map also shows that many respondents believe that the malaria risk zone extends further west and south than is indicated on the governmental malaria risk map, with one respondent including the entirety of South Africa in their rendering of the malaria risk zone. Another respondent included the west coast of the Northern Cape, and many drew lines which either dissected or encompassed Lesotho. This indicates a poor awareness of the government-communicated malaria risk maps of South Africa, and moreover a limited understanding of the relationship between climate and malaria distribution, or at least limited awareness of the climate in these areas.

Overlayed digitized maps of respondent’s malaria boundary drawing indicating the degree of consensus among respondents regarding the location of malaria risk areas in South Africa ( n = 28)
In addition to instructing respondents to map their understanding of malaria risk areas in Southern Africa, the study also sought to assess the accuracy of respondents’ self-reported awareness. Respondents were asked whether, prior to taking the questionnaire, they were aware of malaria risk areas in the region. Of the 32 respondents, the vast majority ( n = 25) indicated that they were aware, while one respondent answered that they were not, and one did not respond. The remaining five respondents stated that they had limited awareness. With such a high degree of self-reported awareness, one would expect more accurate maps than were captured (Fig. (Fig.2). 2 ). Respondents were aware of the general location of malaria risk areas, but could not reliably recount their extent. This indicates a general, and perhaps intuitive, awareness among respondents, but little true, informed awareness.
Interestingly, despite the majority of respondents indicating that they were aware of malaria risk zones, only six felt that there was enough information available to the general public regarding malaria distribution, precautions to take against contracting malaria, and how to identify and treat malaria. Respondents who indicated that they were either aware of malaria risk areas prior to taking the questionnaire, or who indicated that they had some degree of awareness, were then asked to stipulate where they accessed this information. Importantly, government or official publications/websites were the least consulted ( n = 2) source of information, while family, friends, or colleagues were the most frequently cited ( n = 15) sources (Fig. 3 ). Many of the 11 respondents who selected “Other” indicated that they had accessed their information at school, through the media/news, or had been advised by a medical professional (Fig. (Fig.3). 3 ). This could explain the lack of convergence between the government malaria map and those drawn by respondents.

Sources used by respondents to access information regarding malaria distribution in Southern Africa (Respondents were able to select more than one option)
Respondents were asked to rate the level of risk posed by malaria to South African citizens on a Likert scale of 1 to 5, with 1 being low risk, 3 being moderate risk, and 5 being high risk. Respondents most commonly ( n = 11) rated the level of risk as moderate (as can be seen by the single peak), with a slight bias towards higher risk (Fig. 4 ).

Level of risk posed by malaria to South African citizens according to respondents ( n = 32)
B – Avoidance of mosquito B ites
Approximately three quarters ( n = 23) of respondents indicated that they had taken precautions against contracting malaria during their most recent trip to a malaria risk area in Southern Africa, while eight reported taking no precautions and one declined to answer the question. Among the eight respondents who did not take precautions, the most commonly reported reasons were issues around risk assessment and lack of awareness. Interestingly, two respondents indicated that their risk assessment was informed by the advice of locals, with respondent 18 stating:
I heard from people who live there that it was not a risk.
Similarly, respondent 30 reported:
Been travelling to Mozam[bique] for a few years and nothing happened. Locals there also say they moved there and lived there for years – nothing happened.
A further two respondents stated that they were not aware that their destination was a malaria area before departing, and only found out once they returned. For one of these respondents, their destination was Mozambique, a country classified as high risk in its totality. Of the eight respondents who did not take precautions, only one quarter ( n = 2) indicated that they would change their behaviour and employ preventative measures on future trips.
For the 23 respondents who did take precautions, insect repellent was the most popular ( n = 18) preventative measure used (Fig. 5 ). Insect repellent, along with travelling outside of the rainy season, were the two most common precautions used in isolation, each with four respondents. Only 12 respondents used more than one method simultaneously. Both respondents who selected the “Other” option (Fig. (Fig.5) 5 ) cited wearing long trousers and long-sleeved shirts in the evenings. While this would minimize the chances of mosquito bites to the arms and legs, and is effective in reducing the irritation of mosquito bites, the efficacy of clothing worn in the evenings as a method of significantly reducing the risk of contracting malaria remains uncertain (Nakazawa et al. 1998 ; Del Prete et al. 2019 ). Long clothing worn throughout the day and night (Baker, 2018 ), and impregnated with insecticides (Shellvarajah et al. 2017 ), shows more promise, but this is not the approach indicated by the respondents. In this context, wearing long clothing only in the evenings is less effective than these alternatives, and their combined use with chemoprophylaxis (Baker, 2018 ).

Malaria prevention strategies employed by respondents (respondents were able to select more than one option)
Of the 23 respondents who did take precautions against contracting malaria, five indicated that they would only do so again on their next trip to a malaria area if it was in the rainy season/summer. One respondent (respondent 11) stated that they would not take any precautions, as they believed that:
risk is not significantly high when travelling for short time periods
This is a misconception, and studies of imported cases of malaria in Europe and the Middle East resulting from tourists’ short trips to southern Africa underscore this (see Ben-Ami et al. 2005 ; Baranova et al. 2019 ). These erroneous sentiments are particularly concerning given the large proportion of respondents who obtain information on malaria risk from family, friends, and colleagues instead of government publications (Fig. (Fig.3 3 ).
C – C ompliance with C hemoprophylaxis, when indicated
Chemoprophylaxis is indicated for travel to all malaria areas considered moderate to high risk (Schmidt, 2019a , b ). These areas can be found in all of South Africa’s neighbouring countries, and within South Africa in Limpopo and Mpumalanga (Fig. (Fig.1). 1 ). Of the 32 respondents who submitted usable questionnaires, almost all ( n = 30) indicated that they had travelled to malaria areas considered moderate or high risk. However, only one-third ( n = 10) of these respondents reported that they had taken antimalarial drugs on their most recent trip to one of the areas. Even more alarmingly, of the 10 respondents who had only travelled locally within South Africa, to either Limpopo or Mpumalanga, only one-fifth ( n = 2) had taken chemoprophylaxis. This indicates a very low level of compliance with chemoprophylaxis in indicated settings, which is particularly concerning among a sample group who have the level of education and socio-economic status to enable compliance.
Climate change awareness and impact on future malaria risk zones
All 32 respondents indicated they understood what was meant by the term “climate change”. Respondents were then asked whether they thought climate change would impact malaria risk and distribution in Southern Africa, and if so, how. Almost all respondents ( n = 28) indicated that they believed that climate change would increase malaria risk in Southern Africa, while the remaining four indicated that they did not know. No respondents answered that climate change would decrease malaria risk in Southern Africa or have no effect.
Respondents also drew a line across indicating where they thought the boundary of the malaria risk zone would be located in 20 years’ time. The composite map produced shows a far greater extent of malaria distribution than the composite map produced for current malaria risk (Fig. 6 ). Respondents indicated that they believed the malaria risk zone would extend further west and south into the country’s interior, with the majority of respondents including large parts of Limpopo and Mpumalanga (Fig. (Fig.6). 6 ). This is consistent with the trend shown by the malaria risk maps produced by the government over the past century (Coetzee et al. 2013 ; Morris et al. 2013 ). The composite map indicates that respondents are cognizant of the increased risk of malaria projected for South Africa in future decades, and significantly aware of the spatial patterns projected for the increase in the distribution of malaria risk. However, many respondents indicated an expansion of the malaria risk zone 20 years from now which is geographically impossible, and exceeds the model outputs for malaria projection. Notably, the expansion into the mountainous highlands of Lesotho, the temperate climates of the southern coast of South Africa, and the arid west coast of South Africa are unlikely in the next 20 years, if ever. These exaggerated perceptions of future risk, when not realized, may result in further complacency among respondents, particularly as relates to chemoprophylaxis.

Overlaid map of respondents’ perceptions of future malaria risk boundaries in South Africa in 20 years’ time ( n = 28)
Efficacy of the “ABC” of malaria prevention in increasing public awareness
The first tenet of the “ABC” of malaria prevention relates to public awareness and the accurate assessment of malaria risk (NDOH 2018a ). This emphasis on awareness and perception is further echoed in the Malaria Elimination Strategic Plan for South Africa 2019–2023 which aims to “ensure that 90% of the population affected by malaria receives information education communication messaging by 2023” (National Department of Health 2019 :25). When respondents were surveyed, it was found that while there is a relatively high level of self-reported awareness regarding the general location of malaria risk areas in South Africa, the majority of respondents were unable to reliably recount the extent of these areas. The behaviour of the population regarding malaria avoidance similarly was not in line with governmental advice and scientifically determined best practice. This indicates that at least a proportion of the South African population are unaware of the precise location of the malaria risk zone, and their perceived awareness is incongruous with the demonstrated awareness or reality. This is consistent with prior research in KwaZulu-Natal (Maartens et al. 2007 ). Further research is needed to determine whether this lack of awareness in a statistically representative population amounts to greater than 10%.
Within the existing medium to high-risk malaria zones (Fig. (Fig.1), 1 ), targeted governmental efforts at increasing awareness have been conducted actively at the community level, rather than solely passively through websites and risk maps in clinics which is the case for the respondent group from Gauteng Province (Govere et al. 2000 ; Cox et al. 2018 ). For example, members of communities in the Vhembe District in largely rural Limpopo Province who have been involved in the governmental Malaria Awareness Programme (MAP) are found to have a 3.3 times greater knowledge on malaria transmission risk and 2.8 times higher awareness of prevention methods than those who had not been involved in these programmes (Cox et al. 2018 ). The perceptions of residents living in current malaria risk zones regarding the boundary of these zones would be a valuable avenue of future research.
It is notable that among the respondents, government and official publications/websites were the least consulted sources of information, while family, friends, and/or colleagues were the most frequently consulted. As only six of the 32 respondents believed that there was enough information available to the general public regarding malaria distribution, precautions to take against contracting malaria, and how to identify and treat malaria, this suggests a weakness in policy’s strategies regarding modes of delivery of malaria education and highlights the possibility of the transmission of misinformation from person to person. While targeted initiatives in high-risk malaria regions are of course imperative in addressing the epicentre of the problem, the significant tourism sector in South Africa, and the large proportion of people migrating back to their homes in malaria areas during holiday periods, does necessitate a broader geographic reach of these activities (Blumberg et al. 2014 ; Raman et al. 2016 ). Following discrete epidemics, this has been facilitated through tourism operators distributing information (Maartens et al. 2007 ), but this would not address tourists staying in less formalized accommodation. The incorrect perceptions that malaria risk is not high when travelling for short periods (see Ben-Ami et al. 2005 ; Baranova et al. 2019 ), or can be mitigated through wearing long clothing only in the evenings, reveal the dangers of relying on non-official sources of information when planning precautionary measures to avoid malaria (Raman et al. 2016 ). These respondents appear to have misunderstood that while the longer the visit to a malaria-prone region, the greater the risk, short stays do not carry no or low risk; likewise, while long clothing is advised at all times, wearing it for short periods is not sufficient in preventing the risk of bites or malaria (Baker 2018 ). This misconception appears relatively unique to this study; an investigation into foreign tourists’ awareness of malaria while at OR Tambo International Airport in Gauteng indicated that medical practitioners and travel agents were by far the greatest source of information on malaria (Waner et al. 1999 ).
The low rates of use of chemoprophylaxis among respondents warrant particular concern. For persons with no immunity to malaria, which includes our study sample group, but also much of South Africa (Fig. (Fig.1), 1 ), chemoprophylaxis is indicated for the period of travel (Freedman 2008 ; Morris et al. 2013 ; Schmidt, 2019a , b ). Doxycycline, atovaquone-proguanil, and mefloquine are currently recommended for use in South Africa, with an efficacy of ~ 90% when taken correctly (Baker, 2018 ; Schmidt, 2019a , b ). The former two drugs have recently been down-scheduled to S2, allowing for pharmacists to dispense these without prescription (Baker 2018 ), thus increasing access and reducing costs slightly through eliminating the necessity for a consultation with a doctor (Ukpe et al. 2013 ). While there are medical uncertainties regarding the safety of certain antimalarials, particularly Mefloquine, in terms of neuropsychiatric adverse effects (Freedman 2008 ; Baker 2018 ), these were not cited by the respondents as the reasons for non-compliance. More minor side effects, by contrast, were indicated as a reason for non-compliance, a theme which is echoed in studies of international tourists (Waner et al. 1999 ). A lack of awareness of the need for chemoprophylaxis, due to poor knowledge of the malaria areas, or to a lack of understanding of the severity of the risk may explain some of respondents not having used this precaution (Leggat et al. 2002 ; Maartens et al. 2007 ). However, as the majority of respondents over-estimated the expanse of the South African malaria risk area, this seems unlikely. A significant barrier to the use of chemoprophylaxis is the cost (Leggat et al. 2002 ; Ukpe et al. 2013 ). Even among more affluent travellers, the large cost of the antimalarial drugs serves as disincentive, particularly for trips of short duration or outside of the rainfall season (Leggat et al. 2002 ). Greater medical aid coverage for antimalarials and a reduction in the cost of the drugs would be important in addressing this component.
The way forward: policy directions
Despite a relatively low sample size, this study reveals notable gaps in the awareness and risk-taking decision-making among persons who travel into malaria areas, who are resident in a country in which some but not all areas carry a high malaria risk (Blumberg et al. 2014 ). This is distinct to the majority of studies for South Africa which either assess the awareness and behaviour of international tourists visiting malaria areas (cf. Waner et al. 1999 ; Freedman 2008 ), or of local communities within high-risk malaria areas (cf. Govere et al. 2000 ; Morris et al. 2013 ; Cox et al. 2018 ). This group is important because they do not receive the same level of travel advice as international tourists (Waner et al. 1999 ), yet frequently travel into and through high- and medium-risk malaria areas (Maartens et al. 2007 ), often through self-booking (de Jager and Ezeuduji 2015 ). The greatest awareness among local tourists has been found to directly follow major malaria epidemics, which is coupled with booking cancellations for accommodation establishments in malaria-prone regions (Maartens et al. 2007 ). The changes in the methods of malaria risk mapping, and the extent of the risk areas in these maps, particularly under climate change, further yield much of the information that regular travellers might have outdated (Coetzee et al. 2013 ; Morris et al. 2013 ). Policy improvements therefore need to be aware of both the limitations in knowledge and shortfalls in risk-aversion behaviour, and the potential for these factors to worsen under climate change. For effective policy adaptation, however, a much larger sample group with greater socio-economic heterogeneity would be valuable.
While the regularly shifting extent of the malaria risk zone is posited as one of the reasons for a poor awareness among respondents, it could be argued that under climate change, an even more frequent updating and reporting of risk may be beneficial. Seasonal malaria forecasts are being produced for South Africa by local researchers (Kim et al. 2019 ; Landman et al. 2020 ), which could be presented to the public biannually to refine malaria prevention behaviour. Frequent and standardized government publication of season-specific information would potentially result in a greater reliance on these resources over word-of-mouth based on travel which occurred less recently. The avenues of the dissemination are also an important consideration in effective communication of malaria information (Blumberg et al. 2014 ).
Finally, this study was conducted during 2019, prior to the COVID-19 pandemic (Gilbert et al. 2020 ). This pandemic has, both globally and in South Africa, revealed an unprecedented public engagement with disease epidemiology and risk-aversion, and the extensive use of social media in government communication (Gao et al. 2020 ). South Africa has been ranked second in the world for the most reliable COVID-19 information, due in part to the conditions of the national lockdown which prohibit the dissemination of fake news (Ryklief 2020 ). Announcements directly from the National Institute for Communicable Diseases and the Minister of Health, each of which distributed both to the press and via social media, have allowed the public to follow both case numbers and the state of knowledge on precautionary measures. This provides valuable insight into methods of effective communication with the national populace involving disease prevention (Young et al. 2008 ).
This pilot study explores the self-reported risk behaviour and awareness of malaria area boundaries among South Africans who are resident outside of the high-risk malaria area. While respondents in this study claim to have a high level of awareness relating to malaria, the vast majority overestimate the spatial extent of both the current and future malaria areas and do not practice the indicated precautionary measures when visiting regions that they report to understand to be malaria-prone. Part of this disjunct between their understanding of the malaria areas and their behaviour appears to relate to their sources of information, with a considerable reliance on friends and family, rather than official sources. While this pilot study involves a small target group who cannot be considered representative of any broader sub-population, we provide insight as to avenues for more comprehensive research, and a methodology which could be used. This more extensive research would provide valuable insight to improve policy and intervention.
Compliance with ethical standards
Ethics clearance was obtained from the University of the Witwatersrand’s Human Research Ethics Committee (non-medical) prior to entering the field (ethics clearance: gaes-2019-06-01).
- Abiodun GJ, Adebiyi B, Abiodun RO, Oladimeji O, Oladimeji K, Adeola AM, et al. Investigating the resurgence of malaria prevalence in South Africa between 2015 and 2018: a scoping review. Open Health J. 2020; 13 (1):199–125. [ Google Scholar ]
- Adeola A, Ncongwane K, Abiodun G, Makgoale T, Rautenbach H, Botai J, Adisa O, Botai C. Rainfall trends and malaria occurrences in Limpopo Province, South Africa. Int J Env Res Pub He. 2019; 16 (24):5156. doi: 10.3390/ijerph16245156. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Alonso P, Noor AM. The global fight against malaria is at crossroads. Lancet. 2017; 390 (10112):2532–2534. doi: 10.1016/S0140-6736(17)33080-5. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Baker L. Malaria prophylaxis – can we conquer the ‘mighty’ parasite? SA Pharm J. 2018; 85 (2):48–54. [ Google Scholar ]
- Baranova A, Sergiev V, Morozova L, Turbabina N, Morozov E. Imported plasmodium vivax malaria in the Russian federation from Western Sub-Saharan Africa. J Trop Med. 2019; 2019 :1–5. doi: 10.1155/2019/4610498. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ben-Ami R, Siegman-Igra Y, Anis E, Brook GJ, Pitlik S, Dan M, Giladi M. Malaria in travelers returning from short organized tours to holiday resorts in Mombassa, Kenya. Isr Med Assoc J. 2005; 7 :364–367. [ PubMed ] [ Google Scholar ]
- Bernard H. Social research methods: qualitative and quantitative approaches. 2. Los Angeles: SAGE; 2013. [ Google Scholar ]
- Bird D. The use of questionnaires for acquiring information on public perception of natural hazards and risk mitigation – a review of current knowledge and practice. Nat Hazard Earth Sys. 2009; 9 (4):1307–1325. doi: 10.5194/nhess-9-1307-2009. [ CrossRef ] [ Google Scholar ]
- Blumberg L, Frean J, Moonasar D. Successfully controlling malaria in South Africa. SAMJ. 2014; 104 (3):224–227. doi: 10.7196/SAMJ.7600. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Caminade C, Kovats S, Rocklov J, Tompkins A, Morse A, Colón-González F, et al. Impact of climate change on global malaria distribution. PNAS. 2014; 111 (9):3286–3291. doi: 10.1073/pnas.1302089111. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Cella W, Baia-da-Silva DC, Melo GCD, Tadei WP, Sampaio VDS, Pimenta P, Lacerda MVG, Monteiro WM (2019) Do climate changes alter the distribution and transmission of malaria? Evidence assessment and recommendations for future studies. Revista da Sociedade Brasileira de Medicina Tropical 52 [ PubMed ]
- Coetzee M, Kruger P, Hunt R, Durrheim D, Urbach J, Hansford C. Malaria in South Africa: 110 years of learning to control the disease. SAMJ. 2013; 103 (10):770–778. doi: 10.7196/SAMJ.7446. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Cohuet A, Harris C, Robert V, Fontenille D. Evolutionary forces on Anopheles: what makes a malaria vector? Trends Parasitol. 2010; 26 (3):130–136. doi: 10.1016/j.pt.2009.12.001. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Cox SN, Guidera KE, Simon MJ, Nonyane BAS, Brieger W, Bornman MS, Kruger PS. Interactive malaria education intervention and its effect on community participant knowledge: the malaria awareness program in Vhembe district, Limpopo, South Africa. Int Quart Comm Health Edu. 2018; 38 (2):147–158. doi: 10.1177/0272684X17749573. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- De Jager K, Ezeuduji IO. Socio-demographic variables’ relationships in choosing between travel agencies and the internet for leisure travel arrangements: the case of South Africa. Afr J Hosp Tour Leisure. 2015; 4 (2):1–14. [ Google Scholar ]
- Del Prete V, Mateo-Urdiales A, Bueno-Cavanillas A, Ferrara P (2019) Malaria prevention in the older traveller: a systematic review. J Travel Med 26(7). 10.1093/jtm/taz067 [ PubMed ]
- Eikenberry SE, Gumel AB. Mathematics of malaria and climate change. In: Kaper HG, Roberts FS, editors. Mathematics of planet earth. Cham: Springer; 2019. pp. 77–108. [ Google Scholar ]
- Erhardt L, Hobbs FD. Public perceptions of cardiovascular risk in five European countries: the react survey. Int J Clin Pract. 2002; 56 (9):638–644. [ PubMed ] [ Google Scholar ]
- Freedman DO. Malaria prevention in short-term travellers. New Engl J Med. 2008; 359 (6):603–612. doi: 10.1056/NEJMcp0803572. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gao J, Zheng P, Jia Y, Chen H, Mao Y, Chen S, Wang Y, Fu H, Dai J. Mental health problems and social media exposure during COVID-19 outbreak. PlosOne. 2020; 15 (4):e0231924. doi: 10.1371/journal.pone.0231924. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gilbert M, Pullano G, Pinotti F, Valdano E, Poletto C, Boëlle PY, D’Ortenzio E, Yazdanpanah Y, Eholie SP, Altmann M, Gutierrez B, Kraemer MUG, Colizza V. Preparedness and vulnerability of African countries against importations of COVID-19: a modelling study. Lancet. 2020; 395 (10227):871–877. doi: 10.1016/S0140-6736(20)30411-6. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Glik D, Harrison K, Davoudi M, Riopelle D. Public perceptions and risk communications for botulism. Biosecur. 2004; 2 (3):216–223. doi: 10.1089/bsp.2004.2.216. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Goldman RE, Parker DR, Eaton CB, Borkan JM, Gramling R, Cover RT, Ahern DK. Patients’ perceptions of cholesterol, cardiovascular disease risk, and risk communication strategies. Ann Fam Med. 2006; 4 (3):205–212. doi: 10.1370/afm.534. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Govere J, Durrheim D, la Grange K, Mabuza A, Booman M. Community knowledge and perceptions about malaria and practices influencing malaria control in Mpumalanga Province, South Africa. SAMJ. 2000; 90 (6):611–618. [ PubMed ] [ Google Scholar ]
- Kim Y, Ratnam JV, Doi T, Morioka Y, Behera S, Tsuzuki A, et al. Malaria predictions based on seasonal climate forecasts in South Africa: a time series distributed lag nonlinear model. Nat Sci Rep. 2019; 9 (1):1–10. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Landman WA, Sweijd N, Masedi N, Minakawa N. The development and prudent application of climate-based forecasts of seasonal malaria in the Limpopo province in South Africa. Env Dev. 2020; 35 :100522. doi: 10.1016/j.envdev.2020.100522. [ CrossRef ] [ Google Scholar ]
- Leggat PA, Dürrheim DN, Blumberg L. Trends in malaria chemoprophylaxis prescription in South Africa 1994 to 2000. J Trav Med. 2002; 9 (6):318–321. doi: 10.2310/7060.2002.30173. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Maartens F, Sharp B, Curtis B, Mthembu J, Hatting I. The impact of malaria control on perceptions of tourists and tourism operators concerning malaria prevalence in KwaZulu-Natal, 1999/2000 versus 2002/2003. J Trav Med. 2007; 14 (2):96–104. doi: 10.1111/j.1708-8305.2006.00086.x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Maharaj R, Seocharan I, Qwabe B, Mkhabela M, Kissoon S, Lakan V. Decadal epidemiology of malaria in KwaZulu-Natal, a province in South Africa targeting elimination. Malar J. 2019; 18 (1):368. doi: 10.1186/s12936-019-3001-x. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Makinde OS, Abiodun GJ. The impact of rainfall and temperature on malaria dynamics in the KwaZulu-Natal province. South Africa Comm Stat. 2019; 6 :97–108. doi: 10.1080/23737484.2019.1699000. [ CrossRef ] [ Google Scholar ]
- Morris N, Frean J, Baker L, Ukpe I, Barnes K, Kruger P, et al. Re-defining the extent of malaria transmission in South Africa: implications for chemoprophylaxis. SAMJ. 2013; 103 (11):861–864. doi: 10.7196/SAMJ.7533. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Nakazawa M, Ohmae H, Ishii A, Leafasia J. Malaria infection and human behavioral factors: a stochastic model analysis for direct observation data in the Solomon Islands. Am J Hum Biol. 1998; 10 (6):781–789. doi: 10.1002/(SICI)1520-6300(1998)10:6<781::AID-AJHB9>3.0.CO;2-W. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- NDoH . National guidelines for the prevention of malaria, South Africa 2018. Pretoria: National Department of Health; 2018. [ Google Scholar ]
- NDoH (2018b) South African malaria risk map (December 2018. http://wwwnicdacza/wp-content/uploads/2018/12/south_africa_malaria_risk_dec2018_finalpdf
- National Department of Health (2019) Malaria elimination strategy plan for South Africa 2019-2023, Pretoria
- Raman J, Morris N, Frean J, Brooke B, Blumberg L, Kruger P, Mabusa A, Raswiswi E, Shandukani B, Misani E, Groepe MA, Moonasar D. Reviewing South Africa’s malaria elimination strategy (2012–2018): progress, challenges and priorities. Malar J. 2016; 15 (1):438. doi: 10.1186/s12936-016-1497-x. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Roffe SJ, Fitchett JM, Curtis CJ. Classifying and mapping rainfall seasonality in South Africa: a review. S Afr Geogr J. 2019; 101 (2):158–174. doi: 10.1080/03736245.2019.1573151. [ CrossRef ] [ Google Scholar ]
- Ryklief S (2020) South Africa ranks second in the world for reliable Covid-19 news. Independent Online . https://www.iol.co.za/news/south-africa/south-africa-ranks-second-in-the-world-for-reliable-covid-19-news-46850429 .
- Schmidt S. Malaria: the “unwanted souvenir” S Afr Pharm Assist. 2019; 19 (4):8–13. [ Google Scholar ]
- Seale H, Heywood AE, McLaws ML, Ward KF, Lowbridge CP, Van D, et al. Why do I need it? I am not at risk! Public perceptions towards the pandemic (H1N1) 2009 vaccine. BMC Infect Dis. 2010; 10 (1):99. doi: 10.1186/1471-2334-10-99. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Shellvarajah M, Hatz C, Schlagenhauf P. Malaria prevention recommendations for risk groups visiting sub-Saharan Africa: a survey of European expert opinion and international recommendations. Trav Med Infect Dis. 2017; 19 :49–55. doi: 10.1016/j.tmaid.2017.09.002. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Schmidt S. Malaria: the unwanted souvenir. SA Pharm Assist. 2019; 19 (4):8–13. [ Google Scholar ]
- Ukpe IS, Moonasar D, Raman J, Barnes KI, Baker L, Blumberg L. Case management of malaria: treatment and chemoprophylaxis. SAMJ. 2013; 103 (10):793–798. doi: 10.7196/SAMJ.7443. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Waner S, Durrhiem D, Braack LEO, Gammon S. Malaria protection measures used by in-flight travelers to South African game parks. J Trav Med. 1999; 6 (4):254–257. doi: 10.1111/j.1708-8305.1999.tb00528.x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- WHO . Update on the E-2020 initiative of 21 malaria-eliminating countries: report and country briefs. Geneva: World Health Organization; 2018. [ Google Scholar ]
- WHO (2019a) Fact sheet about malaria. https://wwwwhoint/en/news-room/fact-sheets/detail/malaria Accessed 13 March 2019
- WHO . World malaria report. Geneva: World Health Organization; 2019. [ Google Scholar ]
- Young ME, Norman GR, Humphreys KR. Medicine in the popular press: the influence of the media on perceptions of disease. PLoSOne. 2008; 3 (10):e3552. doi: 10.1371/journal.pone.0003552. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
Africa: Mapping Malaria in Africa - Climate Change Study Predicts Where Mosquitoes Will Breed in Future
The relationship between climate and malaria transmission is complex and has been the subject of intense study for some three decades.
Mosquito vector populations sufficient to maintain malaria transmission occur within a particular range of temperatures and humidity that are suitable for their survival and breeding. The parasite also needs suitable temperatures to complete its mosquito life stages. And mosquitoes need surface water to breed in. These conditions have to last long enough for mosquito and parasite populations to grow.
Much of sub-Saharan Africa provides exactly these conditions. Factors like public health interventions, land use, urbanisation and quality of housing also determine transmission and local disease burden. But a suitable climate is a big factor in explaining the latest available data (from 2022). This shows that 94% of the 249 million global malaria cases are recorded in Africa and that nearly all of the 608,000 global malaria deaths annually are on the continent.
Climate change is likely to cause a shift in the suitability for transmission in some areas.
It's fairly straightforward to model the effect of changing temperatures on malaria by using climate data and the thermal ranges of vector and parasite. Rainfall data is less useful, because mosquitoes breed in shallow, slow-moving or standing water, often in very small water bodies such as puddles. And rain doesn't normally stay where it lands. This is where hydrology - the study of water's movement and how it's distributed - becomes useful in modelling.
We are part of an interdisciplinary team that has just published a new set of estimates for future malaria suitability across the African continent in the journal Science. Our work incorporates the dynamics of water flows and stores that can influence breeding locations. The results give a more accurate picture than before of where the malaria transmission season might get longer or shorter as the climate changes.
We found that overall malaria suitability will decrease, especially in west Africa. But other areas, particularly river corridors and floodplains, will become more suitable for malaria transmission. Massive population growth is expected across Africa over the next 25 years, often located close to rivers. This means the number of people living in potentially malaria endemic areas (suitable for transmission more than nine months a year) will increase by 2100 to over a billion.
Along with knowledge of the breeding habitat preferences of specific mosquitoes and their human biting preferences (indoor or outdoor, dusk or night time), this information could help to target and tailor malaria control plans.
Follow the water
Building on our earlier pilot study , published in 2020, in this new study we used seven global hydrological models. Each was run using four climate models. We looked at different possible futures by including a low-, medium- and high-emissions scenario for greenhouse gases.
Read more: Malaria: new map shows which areas will be at risk because of global warming
Thanks to this approach we can now include many hydrological processes such as the soaking of water into the earth, evaporation of water back into the atmosphere and the flow of water through the landscape in large rivers.
This offers the most sophisticated representation yet of potential malaria vector breeding sites across Africa and how these might change in future.
A new picture emerges
Our models paint a complex and realistic pattern of malaria transmission suitability today and in future. Unlike previous work, our model highlights waterways and floodplains as potentially suitable mosquito breeding sites, often on their margins or isolated water bodies nearby.
For instance, the Nile corridor in Egypt is omitted in previous thermal and rainfall models. But when hydrology is included, as in our study, the area is predicted to be highly suitable for malaria transmission.
Egypt is currently malaria-free due to extensive control efforts . It nonetheless remains climatically suitable and malaria mosquitoes can still be found there. We know that malaria was present there until the 1990s and traces of the malaria parasite have even been found in ancient Egyptian mummies .
Read more: From malaria, to smallpox, to polio - here's how we know life in ancient Egypt was ravaged by disease
Changing suitability
Overall, we found that by 2100, a general decrease in malaria suitability is projected across the majority of Africa. Future climates are increasingly either too warm or too dry for year-round malaria transmission.
The main location of this decrease is centred on west Africa around The Gambia. It extends right across the continent at that latitude as far as South Sudan. We also see smaller decreases in suitability in southern Africa around Botswana and Zimbabwe.
This reduction in transmission season length is also seen in previous studies using rainfall to represent surface water. But using hydrological models reveals more concentrated and bigger reductions in season length. The reduced suitability was most pronounced in the high-emissions scenario for greenhouse gases.

Sign up for free AllAfrica Newsletters
Get the latest in African news delivered straight to your inbox
By submitting above, you agree to our privacy policy .
Almost finished...
We need to confirm your email address.
To complete the process, please follow the instructions in the email we just sent you.
There was a problem processing your submission. Please try again later.
Crucially, though, the outcome in our hydrology-driven model was particularly sensitive to future greenhouse gas emissions.
Some areas, particularly around the highlands of Ethiopia, are seen to increase in suitability by 2100, driven by increases in temperature in the cooler mountains. We can also see an increase in malaria suitability following the course of the Orange River in South Africa, where local malaria plans are focused on avoiding re-introduction of transmission along the river.
Is this good news?
Reducing malaria suitability in Africa is a good thing. But when the climate is either too warm or too dry for the malaria parasite or mosquito to survive, there will be other adverse outcomes, particularly for water supply and agriculture. By including water flows in malaria suitability estimates, we can start to examine interactions with these other sectors more directly.
Mark Smith , Professor of Water Science & Heath, University of Leeds
Chris Thomas , Global Professor in Water & Planetary Health, University of Lincoln
This article is republished from The Conversation Africa under a Creative Commons license. Read the original article .
- Environment
Premium Partners
- Africa: OCP Africa Teams Up with Ethiopian Government, the AfDB and Global Partners for Climate-Smart Wheat Initiative (OCP)
- Congo-Brazzaville: Congo Lauds Partnership With African Development Bank, Seeks Enhanced Climate Funding (African Development Bank (AfDB))
AllAfrica publishes around 400 reports a day from more than 100 news organizations and over 500 other institutions and individuals , representing a diversity of positions on every topic. We publish news and views ranging from vigorous opponents of governments to government publications and spokespersons. Publishers named above each report are responsible for their own content, which AllAfrica does not have the legal right to edit or correct.
Articles and commentaries that identify allAfrica.com as the publisher are produced or commissioned by AllAfrica . To address comments or complaints, please Contact us .
AllAfrica is a voice of, by and about Africa - aggregating, producing and distributing 400 news and information items daily from over 100 African news organizations and our own reporters to an African and global public. We operate from Cape Town, Dakar, Abuja, Johannesburg, Nairobi and Washington DC.
- Support our work
- Sign up for our newsletter
- For Advertisers

- © 2024 AllAfrica
- Privacy Policy
You are using an outdated browser. Upgrade your browser today or install Google Chrome Frame to better experience this site.
- Section 2 - Interactions Between Travel Vaccines & Drugs
- Section 2 - Travelers’ Diarrhea
Yellow Fever Vaccine & Malaria Prevention Information, by Country
Cdc yellow book 2024.
Author(s): Mark Gershman, Rhett Stoney (Yellow Fever) Holly Biggs, Kathrine Tan (Malaria)
The following pages present country-specific information on yellow fever (YF) vaccine requirements and recommendations, and malaria transmission information and prevention recommendations. Country-specific maps are included to aid in interpreting the information. The information in this chapter was accurate at the time of publication; however, it is subject to change at any time due to changes in disease transmission or, in the case of YF, changing entry requirements for travelers. Updated information reflecting changes since publication can be found in the online version of this book and on the Centers for Disease Control and Prevention (CDC) Travelers’ Health website. Recommendations for prevention of other travel-associated illnesses can also be found on the CDC Travelers’ Health website .

Yellow Fever Vaccine
Entry requirements.
Entry requirements for proof of YF vaccination under the International Health Regulations (IHR) differ from CDC’s YF vaccination recommendations. Under the IHR, countries are permitted to establish YF vaccine entry requirements to prevent the importation and transmission of YF virus within their boundaries. Certain countries require proof of vaccination from travelers arriving from all countries ( Table 5-25 ); some countries require proof of vaccination only for travelers above a certain age coming from countries with risk for YF virus transmission. The World Health Organization (WHO) defines areas with risk for YF virus transmission as countries or areas where YF virus activity has been reported currently or in the past, and where vectors and animal reservoirs exist.
Unless issued a medical waiver by a yellow fever vaccine provider, travelers must comply with entry requirements for proof of vaccination against YF.
WHO publishes a list of YF vaccine country entry requirements and recommendations for international travelers approximately annually. But because entry requirements are subject to change at any time, health care professionals and travelers should refer to the online version of this book and the CDC Travelers’ Health website for any updates before departure.
CDC Recommendations
CDC’s YF vaccine recommendations are guidance intended to protect travelers from acquiring YF virus infections during international travel. These recommendations are based on a classification system for destination-specific risk for YF virus transmission: endemic, transitional, low potential for exposure, and no risk ( Table 2-08 ). CDC recommends YF vaccination for travel to areas classified as having endemic or transitional risk (Maps 5-10 and 5-11 ). Because of changes in YF virus circulation, however, recommendations can change; therefore, before departure, travelers and clinicians should check CDC’s destination pages for up-to-date YF vaccine information.
Duration of Protection
In 2015, the US Advisory Committee on Immunization Practices published a recommendation that 1 dose of YF vaccine provides long-lasting protection and is adequate for most travelers. The recommendation also identifies specific groups of travelers who should receive additional doses, and others for whom additional doses should be considered (see Sec. 5, Part 2, Ch. 26, Yellow Fever ). In July 2016, WHO officially amended the IHR to stipulate that a completed International Certificate of Vaccination or Prophylaxis is valid for the lifetime of the vaccinee, and YF vaccine booster doses are not necessary. Moreover, countries cannot require proof of revaccination (booster) against YF as a condition of entry, even if the traveler’s last vaccination was >10 years ago.
Ultimately, when deciding whether to vaccinate travelers, clinicians should take into account destination-specific risks for YF virus infection, and individual risk factors (e.g., age, immune status) for serious YF vaccine–associated adverse events, in the context of the entry requirements. See Sec. 5, Part 2, Ch. 26, Yellow Fever , for a full discussion of YF disease and vaccination guidance.
Table 2-08 Yellow fever (YF) vaccine recommendation categories 1
Malaria prevention.
The following recommendations to protect travelers from malaria were developed using the best available data from multiple sources. Countries are not required to submit malaria surveillance data to CDC. On an ongoing basis, CDC actively solicits data from multiple sources, including WHO (main and regional offices); national malaria control programs; international organizations; CDC overseas offices; US military; academic, research, and aid organizations; and the published scientific literature. The reliability and accuracy of those data are also assessed.
If the information is available, trends in malaria incidence and other data are considered in the context of malaria control activities within a given country or other mitigating factors (e.g., natural disasters, wars, the coronavirus disease 2019 pandemic) that can affect the ability to control malaria or accurately count and report it. Factors such as the volume of travel to that country and the number of acquired cases reported in the US surveillance system are also examined. In developing its recommendations, CDC considers areas within countries where malaria transmission occurs, substantial occurrences of antimalarial drug resistance, the proportions of species present, and the available malaria prophylaxis options.
Clinicians should use these recommendations in conjunction with an individual risk assessment and consider not only the destination but also the detailed itinerary, including specific cities, types of accommodations, season, and style of travel, as well as special health conditions (e.g., pregnancy). Several medications are available for malaria prophylaxis. When deciding which drug to use, consider the itinerary and length of trip, travelers’ previous adverse reactions to antimalarials, drug allergies, medical history, and drug costs. For a thorough discussion of malaria and guidance for prophylaxis, see Sec. 5, Part 3, Ch. 16, Malaria .
South Africa
Entry requirements : Required for travelers ≥1 year old arriving from countries with risk for YF virus transmission; this includes >12-hour airport transits or layovers in countries with risk for YF virus transmission. 1
CDC recommendations : Not recommended
- Along the border with Mozambique and Zimbabwe
- KwaZulu-Natal Province: uMkhanyakude District; the districts of King Cetshwayo and Zululand (few cases) Limpopo Province: the districts of Mopani and Vhembe; the districts of Capricorn, Greater Sekhukhune, and Waterberg (few cases)
- Mpumalanga Province: Ehlanzeni District
- Kruger National Park
- Chloroquine
- P. falciparum (primarily)
- P. malariae , P. ovale , and P. vivax (less commonly)
- KwaZulu-Natal Province (uMkhanyakude District); Limpopo Province (the districts of Mopani and Vhembe); Mpumalanga Province (Ehlanzeni District); and Kruger National Park: Atovaquone-proguanil, doxycycline, mefloquine, tafenoquine 3
- All other areas with malaria transmission (including the districts of King Cetshwayo and Zululand in KwaZulu-Natal Province, and the districts of Capricorn, Greater Sekhukhune, and Waterberg in Limpopo Province): No chemoprophylaxis recommended (insect bite precautions and mosquito avoidance only) 4
Related Maps
Map 2-15 malaria prevention in south africa, other vaccines to consider.
See Health Information for Travelers to South Africa .
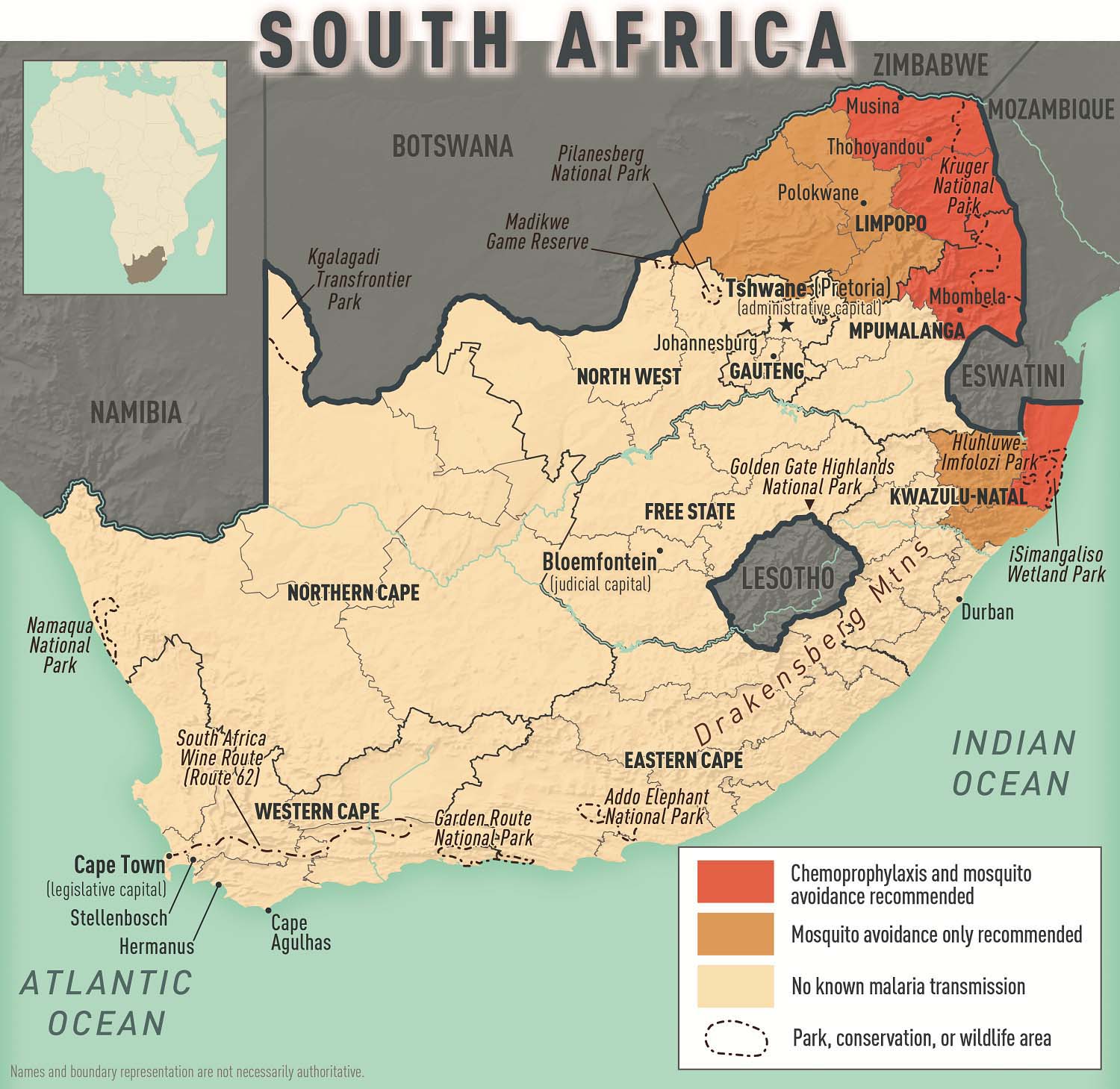
View Larger
1 Current as of November 2022. This is an update of the 2010 map created by the Informal WHO Working Group on the Geographic Risk of Yellow Fever.
2 Refers to Plasmodium falciparum malaria, unless otherwise noted.
3 Tafenoquine can cause potentially life-threatening hemolysis in people with glucose-6-phosphate-dehydrogenase (G6PD) deficiency. Rule out G6PD deficiency with a quantitative laboratory test before prescribing tafenoquine to patients.
4 Mosquito avoidance includes applying topical mosquito repellant, sleeping under an insecticide-treated mosquito net, and wearing protective clothing (e.g., long pants and socks, long-sleeve shirt). For additional details on insect bite precautions, see Sec. 4, Ch. 6, Mosquitoes, Ticks & Other Arthropods.
5 Primaquine can cause potentially life-threatening hemolysis in people with G6PD deficiency. Rule out G6PD deficiency with a quantitative laboratory test before prescribing primaquine to patients.
6 P. knowlesi is a malaria species with a simian (macaque) host. Human cases have been reported from most countries in Southwest Asia and are associated with activities in forest or forest-fringe areas. P. knowlesi has no known resistance to antimalarials.
Yellow Fever Maps
2 In 2017, the Centers for Disease Control and Prevention (CDC) expanded its YF vaccination recommendations for travelers going to Brazil because of a large YF outbreak in multiple states in that country. Please refer to the CDC Travelers’ Health website for more information and updated recommendations.
3 YF vaccination is generally not recommended for travel to areas where the potential for YF virus exposure is low. Vaccination might be considered, however, for a small subset of travelers going to these areas who are at increased risk for exposure to YF virus due to prolonged travel, heavy exposure to mosquitoes, or inability to avoid mosquito bites. Factors to consider when deciding whether to vaccinate a traveler include destination-specific and travel-associated risks for YF virus infection; individual, underlying risk factors for having a serious YF vaccine–associated adverse event; and destination entry requirements.
The following authors contributed to the previous version of this chapter: Mark D. Gershman, Emily S. Jentes, Rhett J. Stoney (Yellow Fever) Kathrine R. Tan, Paul M. Arguin (Malaria)
File Formats Help:
- Adobe PDF file
- Microsoft PowerPoint file
- Microsoft Word file
- Microsoft Excel file
- Audio/Video file
- Apple Quicktime file
- RealPlayer file
- Zip Archive file
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.
A .gov website belongs to an official government organization in the United States.
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- How It Spreads
- Where Malaria Occurs
- World Malaria Day 2024
- Clinical Guidance: Malaria Diagnosis & Treatment in the U.S.
- Clinical Features
- Clinical Testing and Diagnosis
- Malaria Risk Assessment for Travelers
- Choosing a Drug to Prevent Malaria
- How to Report a Case of Malaria
- Public Health Strategy
- Malaria's Impact Worldwide
- Communication Resources
Preventing Malaria While Traveling
- You can take medications to prevent malaria.
- Check to see if malaria spreads in the region or country you will be visiting before you travel.
- Take medications to prevent malaria as prescribed, including the period before travel and after you return from travel.
- Avoid mosquito bites even if you are taking medications to prevent malaria.
- If you experience symptoms of malaria, especially fever, while traveling or after returning, seek immediate medical attention.

This information is for travelers who live in the United States. Travelers from other countries should consult healthcare providers in their country for information on prevention recommendations and availability of antimalarial drugs. For more health recommendations for international travel, visit the CDC Yellow Book .
Every year, millions of U.S. residents travel to countries where malaria is present. About 2,000 cases of malaria are diagnosed in the U.S. in a typical year, mostly in returned travelers. You can prevent malaria when travelling in areas where malaria spreads by taking medications, called antimalarials, and preventing mosquito bites. There is no vaccine for malaria currently available in the U.S.
Risk factors
Malaria does not regularly occur in the in the U.S., so there is usually no exposure to the disease here. Travelers, especially to sub-Saharan Africa, regions of South America and Southeast Asia, have the greatest risk of getting malaria and potentially dying from their infection if not diagnosed promptly and appropriately treated. All travelers to countries where malaria is present may be at risk for infection.
U.S.-based travelers going to an area where malaria spreads, even if they had some level of immunity to malaria in the past, are still at risk for infection. It's important to note that acquired immunity to (protection from) malaria weakens the longer you are away from an area where malaria is widespread or endemic. If you have been away from your country of origin, even a short time, you can lose your protective immunity very quickly and should use the same preventative measures as all travelers (preventative medication, mosquito bite prevention, etc.)
Prevention steps
Before you travel.
Before you travel, learn about the health risks and precautions for malaria and other diseases for your destination . Get a detailed itinerary of all the possible destinations or places you may visit during the trip. Check to see if malaria is present and spreads in these locations. CDC's Yellow Book chapter on Malaria Prevention by Country provides detailed information about the specific parts of countries where malaria spreads. It also provides additional information including the species of malaria that occur there, if there is resistance to any of the antimalarial drugs, and the specific medicines that CDC recommends for use for malaria prevention in each country or region.
Resource
Understand your risk.
To understand your level of risk for getting malaria and if you need to take malaria prevention medications, consider the following
- Destination country or region
- Specific itinerary, including specific cities, types of accommodation (hotel with AC or open-air tents), season, and style of travel
- Pregnancy status, other medical conditions, and current medications you take
- Antimalarial drug resistance at your destination
Choose the most appropriate malaria prevention measures
You should discuss with a healthcare provider a detailed itinerary of where you are traveling, your activities, accommodations, and your medical history to understand your risk of malaria and if you need to take medication to prevent malaria.
- There are various options of medications available to prevent malaria and based on the country/countries, the urgency of the trip, and how often you prefer to take medication (daily vs. weekly).
- If your provider recommends malaria prevention medications, it is important to take them as prescribed, including the period before travel and after you return from travel.
- In some areas where only a few cases of malaria occur, CDC recommends preventing mosquito bites as the only way to prevent malaria.
- Be aware of counterfeit (fake) or substandard (not made according to U.S. standards) drugs sold in some countries. This includes countries where malaria spreads. These drugs may not be effective. Get all your medications, including antimalarial drugs, in the US, before traveling overseas.
Healthcare providers can reference the Risk Assessment page to ensure they provide appropriate recommendation for all travelers at risk for malaria, including those returning to visit friends and relative.
Additional steps to take while traveling
It is important to take steps to avoid mosquito bites even if you are taking medications as an added layer to prevent malaria.
Steps to prevent mosquito bites
- DEET (Insect repellents that contain DEET offer the best protection against mosquito bites.)
- Picaridin (known as KBR 3023 and icaridin outside the US)
- Oil of lemon eucalyptus (OLE)
- Para-menthane-diol (PMD)
- 2-undecanon
- Wear loose-fitting, long-sleeved shirts and pants and socks.
- Use 0.5% permethrin spray to treat clothing and gear (such as boots, pants, socks, and tents) or buy permethrin-treated clothing and gear.
- Do not use permethrin products directly on skin.
- Keep windows and doors closed or covered with screens to keep mosquitoes out of your house.
- Repair broken screening on windows, doors, porches, and patios.
- Sleep in a well-screened or air-conditioned room, or sleep under a permethrin-treated bed net.
Know the symptoms of malaria
Although malaria prevention strategies can be very effective, none will protect 100% of the time. Malaria is always a serious disease and may cause death. If you have a fever or flu-like illness either while traveling in an area where malaria spreads or after returning home (for up to one year) seek immediate medical attention. Tell your healthcare provider about your travel history.
When immediate care is needed
Blood donation eligibility.
If you recently traveled to an area where malaria is widespread, be sure to understand if you can donate blood .
Malaria is a serious disease caused by a parasite that infects the Anopheles mosquito. You get malaria when bitten by an infective mosquito.
For Everyone
Health care providers, public health.
This website uses cookies to ensure you get the best experience on our website. Learn more

Information on how to stay safe and healthy abroad. About us.
- Destinations
- Asia (Central)
- Asia (East)
- Australasia & Pacific
- Central America
- Europe & Russia
- Middle East
- North America
- South America & Antarctica
Kenya Malaria Map
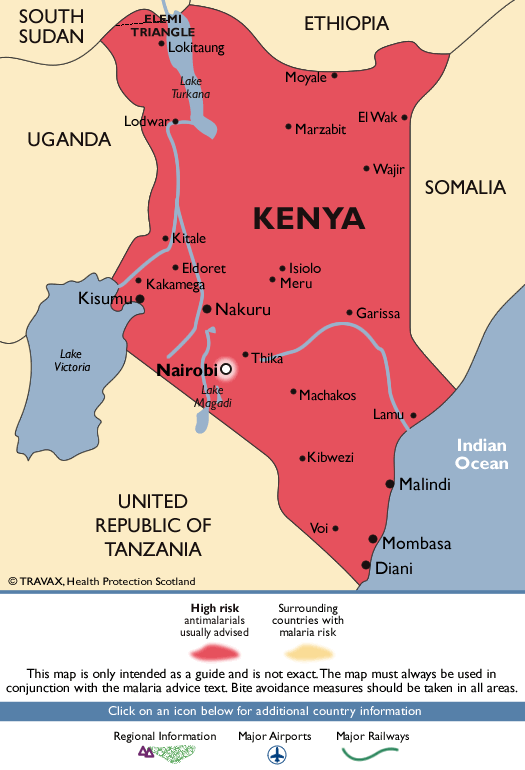
This website uses cookies to ensure you get the best experience on our website. Learn more

Information on how to stay safe and healthy abroad. About us.
- Destinations
- Asia (Central)
- Asia (East)
- Australasia & Pacific
- Central America
- Europe & Russia
- Middle East
- North America
- South America & Antarctica
Congo Malaria Map

IMAGES
VIDEO
COMMENTS
Map showing extent of malaria risk in South Africa. This website uses cookies to ensure you get the best experience on our website.
South Africa Malaria Map
Advice for All Destinations COVID-19. Read the information on the COVID-19: Health Considerations for Travel page for advice on travelling during the COVID-19 pandemic.. Vaccinations and malaria risk. Review both the Vaccination and Malaria sections on this page to find out if you may need vaccines and/or a malaria risk assessment before you travel to this country.
South Africa Malaria Map. © 2024 - My ASP.NET Application
Dosing info - Hep B. Malaria. CDC recommends that travelers going to certain areas of South Africa take prescription medicine to prevent malaria. Depending on the medicine you take, you will need to start taking this medicine multiple days before your trip, as well as during and after your trip.
PowerPoint Presentation. BOTSWANA 16 Alldays Swartwater ZIMBABWE Musina Vhembe SOUTH AFRICAN MALARIA RISK MAP DECEMBER 2018 To significantly reduce your risk, take precautionary measures against mosquito bites throughout the year in ALL RISK areas Where malaria chemoprophylaxis "s indicated, mefloquine or atovaquone-proguanil or doxycycline ...
Malaria in humans is caused by protozoan parasites of the genus Plasmodium, including Plasmodium falciparum, P. malariae, P. ovale, and P. vivax. In addition, zoonotic forms have been documented as causes of human infections and some deaths, especially P. knowlesi, a parasite of Old World (Eastern Hemisphere) monkeys, in Southeast Asia.
M A P 2 -2 5. Malaria in South Africa. Windhoek NAMIBIA Alexander Springbok Vanhynsdorp BOTSWANA Gaborone Mmabatho NORTH WEST Musina MOZAMBIOU Polokwane. Maputo GAUTENG WAZIL D Vereeniging Johannesburg Vryburg. NORTHERN CAPE Kimberley. Upington SOU AFRICA De Aar Klerksdorp.
The Malaria Threats Map is a comprehensive platform on the four biological threats to malaria control and elimination. ... Gene deletions among some malaria parasites cause false negative diagnostic test results, complicating case management and control. Number of studies: 333. Database last updated: 5/19/2024.
Reproduced with permission: The National Department of Health, Republic of South Africa and the South African Malaria Elimination Committee. This map and key should be used with the description of malaria risk areas and recommendations as provided on TravelHealthPro malaria page as above. Click on map to open in a new window; Recommended ...
This image shows a map with the countries of South Africa. Regions with a low risk of malaria are marked in light pink, regions with a moderate risk are marked in orange, regions with a high risk are marked in red and regions with a seasonal risk are marked in purple. This information, and the recommended preventive measures, can be looked up ...
South America, Asia and Oceania(WHO 2016). In the present-day South Africa, malaria transmission occurs in the north-eastern part of the country, mainly in the low altitude (below 1 000m above sea-level) areas of Limpopo, Mpumalanga and northern KwaZulu-Natal. (See map of malaria risk areas in South Africa, Pg. 40).
South Africa's latitude spans 22°S to 34°S, and its elevation ranges from sea level to 3,482 m (≈11,500 ft), although the average height of Highveld plateau in the interior of the country is around 1,200 m (≈4000 ft). In some areas of South Africa (e.g., Durban, Pretoria), the UV index exceeds 11 in the summer months, which is ...
3. Malaria Statistics in South Africa. Over the past decades, South Africa has shown consistent improvement in reducing both the morbidity and mortality rates associated with malaria [].For many years, annual malaria cases in South Africa were maintained below 10,000 due to vector control and case management efforts, with approximately 8750 reported cases in 1995 [].
This shows that 94% of the 249 million global malaria cases are recorded in Africa and that nearly all of the 608,000 global malaria deaths annually are on the continent.
Methods. Malaria can be found in each of South Africa's neighbouring countries, barring Lesotho, where the altitude and consequent colder temperatures make it inhospitable to the malaria-carrying Anopheles mosquito (Blumberg et al. 2014).Within South Africa itself, malaria is confined to the north-eastern reaches of the country, where it is endemic to three of South Africa's nine provinces ...
Reprint of malaria map from Health Information for International Travel 1974 (CDC 1974) View Larger Figure. For many years, CDC Yellow Book included World Health Organization global malaria maps, which generally followed the above design style. Small size and lack of labels made these maps difficult to interpret for specific travel itineraries.
Malaria is a climate-sensitive, vector-borne disease that caused 619,000 deaths among 247 million cases in 2021 ( 1 ). This burden is acutely focused on Africa, where 95% of global cases are reported ( 1 ). Reductions in cases in Africa have slowed or even reversed in recent years, attributed in part to a stall in investments in global ...
Map showing extent of malaria risk in Namibia.
This shows that 94% of the 249 million global malaria cases are recorded in Africa and that nearly all of the 608,000 global malaria deaths annually are on the continent. ... Malaria: new map ...
CDC Yellow Book 2024. Author (s): Mark Gershman, Rhett Stoney (Yellow Fever) Holly Biggs, Kathrine Tan (Malaria) The following pages present country-specific information on yellow fever (YF) vaccine requirements and recommendations, and malaria transmission information and prevention recommendations. Country-specific maps are included to aid in ...
Every year, millions of U.S. residents travel to countries where malaria is present. About 2,000 cases of malaria are diagnosed in the U.S. in a typical year, mostly in returned travelers. You can prevent malaria when travelling in areas where malaria spreads by taking medications, called antimalarials, and preventing mosquito bites.
Map showing extent of malaria risk in Kenya.
Tswalu, which is South Africa's largest private game reserve, spans over an incredible 282,000 acres — making it nearly double the size of Chicago — and it pulsates with possibilities. Even ...
Map showing extent of malaria risk in Congo.